Neurogenesis-dependent antidepressant-like activity of Hericium erinaceus in an animal model of depression
- PMID: 34876186
- PMCID: PMC8650354
- DOI: 10.1186/s13020-021-00546-8
Neurogenesis-dependent antidepressant-like activity of Hericium erinaceus in an animal model of depression
Abstract
Background: Depression is a severe neuropsychiatric disorder that affects more than 264 million people worldwide. The efficacy of conventional antidepressants are barely adequate and many have side effects. Hericium erinaceus (HE) is a medicinal mushroom that has been reported to have therapeutic potential for treating depression.
Methods: Animals subjected to chronic restraint stress were given 4 weeks HE treatment. Animals were then screened for anxiety and depressive-like behaviours. Gene and protein assays, as well as histological analysis were performed to probe the role of neurogenesis in mediating the therapeutic effect of HE. Temozolomide was administered to validate the neurogenesis-dependent mechanism of HE.
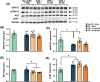
Results: The results showed that 4 weeks of HE treatment ameliorated depressive-like behaviours in mice subjected to 14 days of restraint stress. Further molecular assays demonstrated the 4-week HE treatment elevated the expression of several neurogenesis-related genes and proteins, including doublecortin, nestin, synaptophysin, brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinase B (TrkB), phosphorylated extracellular signal-regulated kinase, and phosphorylated cAMP response element-binding protein (pCREB). Increased bromodeoxyuridine-positive cells were also observed in the dentate gyrus of the hippocampus, indicating enhanced neurogenesis. Neurogenesis blocker temozolomide completely abolished the antidepressant-like effects of HE, confirming a neurogenesis-dependent mechanism. Moreover, HE induced anti-neuroinflammatory effects through reducing astrocyte activation in the hippocampus, which was also abolished with temozolomide administration.
Conclusion: HE exerts antidepressant effects by promoting neurogenesis and reducing neuroinflammation through enhancing the BDNF-TrkB-CREB signalling pathway.
Keywords: Anti-neuroinflammatory; Depression; Hericium erinaceus; Hippocampus; Neurogenesis.
© 2021. The Author(s).
Conflict of interest statement
Ganofarm R&D Sdn Bhd supplied the standardised extract of HE (Memorandum of Understanding for Academic Cooperation between Universiti Malaya and Ganofarm R&D Sdn Bhd dated 20 August 2018). The authors declare that the research was conducted in the absence of any commercial or financial relationships that may bias the data interpretation and scientific output. This company had no role in the study design and conceptualisation, data collection/analysis, interpretation, and scientific writing of this work.
Figures








References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. - PMC - PubMed
-
- Jokela M, Virtanen M, Batty GD, Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiat. 2016;73(1):87–88. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

