Structural MRI profiles and tau correlates of atrophy in autopsy-confirmed CTE
- PMID: 34876229
- PMCID: PMC8653514
- DOI: 10.1186/s13195-021-00928-y
Structural MRI profiles and tau correlates of atrophy in autopsy-confirmed CTE
Abstract
Background: Chronic traumatic encephalopathy (CTE), a neurodegenerative tauopathy, cannot currently be diagnosed during life. Atrophy patterns on magnetic resonance imaging could be an effective in vivo biomarker of CTE, but have not been characterized. Mechanisms of neurodegeneration in CTE are unknown. Here, we characterized macrostructural magnetic resonance imaging features of brain donors with autopsy-confirmed CTE. The association between hyperphosphorylated tau (p-tau) and atrophy on magnetic resonance imaging was examined.
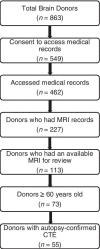
Methods: Magnetic resonance imaging scans were obtained by medical record requests for 55 deceased symptomatic men with autopsy-confirmed CTE and 31 men (n = 11 deceased) with normal cognition at the time of the scan, all >60 years Three neuroradiologists visually rated regional atrophy and microvascular disease (0 [none]-4 [severe]), microbleeds, and cavum septum pellucidum presence. Neuropathologists rated tau severity and atrophy at autopsy using semi-quantitative scales.
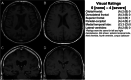
Results: Compared to unimpaired males, donors with CTE (45/55=stage III/IV) had greater atrophy of the orbital-frontal (mean diff.=1.29), dorsolateral frontal (mean diff.=1.31), superior frontal (mean diff.=1.05), anterior temporal (mean diff.=1.57), and medial temporal lobes (mean diff.=1.60), and larger lateral (mean diff.=1.72) and third (mean diff.=0.80) ventricles, controlling for age at scan (ps<0.05). There were no effects for posterior atrophy or microvascular disease. Donors with CTE had increased odds of a cavum septum pellucidum (OR = 6.7, p < 0.05). Among donors with CTE, greater tau severity across 14 regions corresponded to greater atrophy on magnetic resonance imaging (beta = 0.68, p < 0.01).
Conclusions: These findings support frontal-temporal atrophy as a magnetic resonance imaging finding of CTE and show p-tau accumulation is associated with atrophy in CTE.
Keywords: Atrophy; Chronic traumatic encephalopathy; Magnetic resonance imaging; Neurodegeneration; Tau.
© 2021. The Author(s).
Conflict of interest statement
Lee E. Goldstein is a paid consultant to Johnson & Johnson (New Brunswick, NJ) / Janssen Research & Development, LLC (Raritan, NJ) and Rebiscan, Inc. (Cambridge, MA). He has received funding from the WWE and Ivivi Health Sciences. Robert A. Stern is a member of the Mackey-White Committee of the NFL Players Association. He is a paid consultant to Biogen (Cambridge, MA, USA). He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA), and is a member of the Board of Directors of King-Devick Technologies (Chicago, IL, USA). Robert C. Cantu is a senior advisor to the NFL Head Neck & Spine Committee, VP of NOCSAE and Chair Scientific Advisory Committee, Co-Founder and Medical Director of the Concussion Legacy Foundation; receives royalties from Houghton Mifflin Harcourt; receives compensation for legal expert opinion (NCAA, NHL etc.); and is on the medical science committee for the National Collegiate Athletic Association Student-Athlete Concussion Injury Litigation. Ann C. McKee is a member of the Mackey-White Committee of the NFL Players Association. Chris Nowinski is a member of the Mackey-White committee of the NFL Players Association and a shareholder in Oxeia Biopharmaceuticals. Rhoda Au is a scientific advisor to Signant Health and Biogen.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- AG054156/AG/NIA NIH HHS/United States
- U54NS115266/NS/NINDS NIH HHS/United States
- K23 NS102399/NS/NINDS NIH HHS/United States
- U54 NS115266/NS/NINDS NIH HHS/United States
- AG016495/AG/NIA NIH HHS/United States
- I01 CX001038/CX/CSRD VA/United States
- AG008122/AG/NIA NIH HHS/United States
- AG049810/AG/NIA NIH HHS/United States
- RF1 AG057902/AG/NIA NIH HHS/United States
- K23AG046377/AG/NIA NIH HHS/United States
- RF1AG054156/AG/NIA NIH HHS/United States
- U01NS086659/NS/NINDS NIH HHS/United States
- RF1 AG062109/AG/NIA NIH HHS/United States
- U01 NS086659/NS/NINDS NIH HHS/United States
- P30AG072978/AG/NIA NIH HHS/United States
- K23NS102399/NS/NINDS NIH HHS/United States
- R56 AG062109/AG/NIA NIH HHS/United States
- AG062109/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

