Comparison of cisplatin-induced anti-tumor response in CT26 syngeneic tumors of three BALB/c substrains
- PMID: 34876239
- PMCID: PMC8653566
- DOI: 10.1186/s42826-021-00110-3
Comparison of cisplatin-induced anti-tumor response in CT26 syngeneic tumors of three BALB/c substrains
Abstract
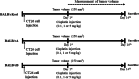
Background: To determine whether the background of BALB/c substrains affects the response to anti-tumor drugs, we measured for alterations in tumor growth, histopathological structure of the tumor, and expressions of tumor-related proteins in three BALB/c substrains derived from different sources (BALB/cKorl, BALB/cA and BALB/cB), after exposure to varying concentrations of cisplatin (0.1, 1 and 5 mg/kg).
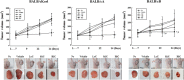
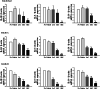
Results: Cisplatin treatment induced similar responses for body and organ weights, serum analyzing factors, and blood analyzing factors in all BALB/c substrains with CT26 syngeneic tumor. Few differences were detected in the volume and histopathological structure of the CT26 tumor. Growth inhibition of CT26 tumors after exposure to cisplatin was greater in the BALB/cB substrain than BALB/cKorl and BALB/cA substrains, and a similar pattern was observed in the histopathological structure of tumors. However, the expression levels of other tumor-related factors, including Ki67, p27, p53, Bcl-2-associated X protein (Bax), B-cell lymphoma 2 (Bcl-2), caspase-3 (Cas-3), matrix metallopeptidase 2 (MMP2) and vascular endothelial growth factor (VEGF) proteins, were constantly maintained in the tumors of all three substrains after cisplatin treatment. A similar decrease pattern was observed for the expressions of inflammatory cytokines, including interleukin (IL)-1β, IL-6 and IL-10, in the CT26 tumors of the three BALB/c substrains.
Conclusions: Taken together, results of the present study indicate that the genetic background of the three BALB/c substrains has no major effect on the therapeutic responsiveness of cisplatin, except growth and histopathology of the CT26 syngeneic tumor.
Keywords: BALB/c; BALB/cKorl; CT26 colon cancer cell; Cisplatin; Substrains.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

