Sterile tuberculous granuloma in a patient with XDR-TB treated with bedaquiline, pretomanid and linezolid
- PMID: 34876446
- PMCID: PMC8655514
- DOI: 10.1136/bcr-2021-245612
Sterile tuberculous granuloma in a patient with XDR-TB treated with bedaquiline, pretomanid and linezolid
Abstract
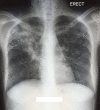
Drug-resistant tuberculosis (DR-TB) continues to pose a threat to the global eradication of TB. Regimens for extensively drug-resistant (XDR) TB are lengthy and poorly tolerated, often with unsuccessful outcomes. The TB Alliance Nix-TB trial investigated the safety and efficacy of a 26-week regimen of bedaquiline, pretomanid and linezolid (BPaL) in participants with XDR-TB, multidrug-resistant (MDR) TB treatment failure or intolerance. In this trial 9 out of 10 participants were cured. We describe a trial participant with XDR-TB who presented with new-onset seizures soon after BPaL treatment completion. Imaging showed a right temporal ring-enhancing lesion, and a sterile tuberculous granuloma was confirmed after a diagnostic, excisional biopsy. Learning points include management of a participant with a tuberculoma after BPaL completion, efficacy of new medications for central nervous system (CNS) TB and a review of their CNS penetration. This is the first case of pretomanid use in CNS TB.
Keywords: TB and other respiratory infections; drugs: CNS (not psychiatric); drugs: infectious diseases; infection (neurology); medical management.
© BMJ Publishing Group Limited 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: PH: trial sponsor paid for article processing charges. Funding paid to institution for conduct of trial related research. CU: member of executive group for the SimpliciTB trial co-funded by TB Alliance (trial sponsor). Funding paid to institution for conduct of trial related research. NM: funding paid to institution for conduct of trial related research. MO: Employee of TB Alliance.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
