Distinct Contribution of Granular and Agranular Subdivisions of the Retrosplenial Cortex to Remote Contextual Fear Memory Retrieval
- PMID: 34876468
- PMCID: PMC8808736
- DOI: 10.1523/JNEUROSCI.1303-21.2021
Distinct Contribution of Granular and Agranular Subdivisions of the Retrosplenial Cortex to Remote Contextual Fear Memory Retrieval
Abstract
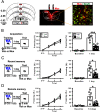
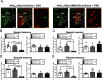
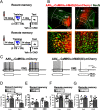
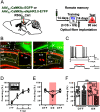
The retrieval of recent and remote memories are thought to rely on distinct brain circuits and mechanisms. The retrosplenial cortex (RSC) is robustly activated during the retrieval of remotely acquired contextual fear memories (CFMs), but the contribution of particular subdivisions [granular (RSG) vs agranular retrosplenial area (RSA)] and the circuit mechanisms through which they interact to retrieve remote memories remain unexplored. In this study, using both anterograde and retrograde viral tracing approaches, we identified excitatory projections from layer 5 pyramidal neurons of the RSG to the CA1 stratum radiatum/lacunosum-moleculare of the dorsal hippocampus and the superficial layers of the RSA in male mice. We found that chemogenetic or optogenetic inhibition of the RSG-to-CA1, but not the RSG-to-RSA, pathway selectively impairs the retrieval of remote CFMs. Collectively, our results uncover a specific role for the RSG in remote CFM recall and provide circuit evidence that RSG-mediated remote CFM retrieval relies on direct RSG-to-CA1 connectivity. The present study provides a better understanding of brain circuit mechanisms underlying the retrieval of remote CFMs and may help guide the development of therapeutic strategies to attenuate remote traumatic memories that lead to mental health issues such as post-traumatic stress disorder.SIGNIFICANCE STATEMENT The RSC is implicated in contextual information processing and remote recall. However, how different subdivisions of the RSC and circuit mechanisms through which they interact to underlie remote memory recall remain unexplored. This study shows that granular subdivision of the RSC and its input to hippocampal area CA1 contributes to the retrieval of remote contextual fear memories. Our results support the hypothesis that the RSC and hippocampus require each other to preserve fear memories and may provide a novel therapeutic avenue to attenuate remote traumatic memories in patients with post-traumatic stress disorder.
Keywords: CA1; agranular retrosplenial cortex; contextual fear memory; granular retrosplenial cortex; memory retrieval; remote memory.
Copyright © 2022 the authors.
Figures










References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
