Disruption of VGLUT1 in Cholinergic Medial Habenula Projections Increases Nicotine Self-Administration
- PMID: 34876472
- PMCID: PMC8751853
- DOI: 10.1523/ENEURO.0481-21.2021
Disruption of VGLUT1 in Cholinergic Medial Habenula Projections Increases Nicotine Self-Administration
Abstract
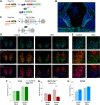
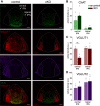
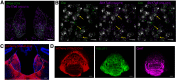
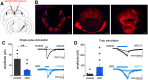
Cholinergic projections from the medial habenula (MHb) to the interpeduncular nucleus (IPN) have been studied for their complex contributions to nicotine addiction and have been implicated in nicotine reinforcement, aversion, and withdrawal. While it has been established that MHb cholinergic projections corelease glutamate, no direct evidence has demonstrated a role for this glutamate projection in nicotine consumption. In the present study, a novel floxed Slc17a7 [vesicular glutamate transporter 1 (VGLUT1)] mouse was generated and used to create conditional knock-out (cKO) mice that lack VGLUT1 in MHb cholinergic neurons. Loss of Slc17a7 expression in ventral MHb cholinergic neurons was validated using fluorescent in situ hybridization, and immunohistochemistry was used to demonstrate a corresponding reduction of VGLUT1 protein in cholinergic terminals in the IPN. We also used optogenetics-assisted electrophysiology to evoke EPSCs in IPN and observed a reduction of glutamatergic currents in the cKO, supporting the functional disruption of VGLUT1 in MHb to IPN synapses. cKO mice exhibited no gross phenotypic abnormalities and displayed normal thigmotaxis and locomotor behavior in the open-field assay. When trained to lever press for food, there was no difference between control and cKO. However, when tested in a nicotine self-administration procedure, we found that the loss of VGLUT1-mediated glutamate corelease led to increased responding for nicotine. These findings indicate that glutamate corelease from ventral MHb cholinergic neurons opposes nicotine self-administration, and provide additional support for targeting this synapse to develop potential treatments for nicotine addiction.
Keywords: acetylcholine; corelease; glutamate; interpeduncular nucleus; medial habenula; nicotine.
Copyright © 2022 Souter et al.
Figures
References
-
- Akers AT, Cooper SY, Baumgard ZJ, Casinelli GP, Avelar AJ, Henderson BJ (2020) Upregulation of nAChRs and changes in excitability on VTA dopamine and GABA neurons correlates to changes in nicotine-reward-related behavior. eNeuro 7:ENEURO.0189-20.2020–0120. 10.1523/ENEURO.0189-20.2020 - DOI - PMC - PubMed
-
- Antolin-Fontes B, Li K, Ables JL, Riad MH, Görlich A, Williams M, Wang C, Lipford SM, Dao M, Liu J, Molina H, Heintz N, Kenny PJ, Ibañez-Tallon I (2020) The habenular G-protein–coupled receptor 151 regulates synaptic plasticity and nicotine intake. Proc Natl Acad Sci USA 117:5502–5509. 10.1073/pnas.1916132117 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
