Microtopographical guidance of macropinocytic signaling patches
- PMID: 34876521
- PMCID: PMC8685668
- DOI: 10.1073/pnas.2110281118
Microtopographical guidance of macropinocytic signaling patches
Abstract
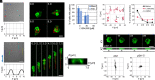
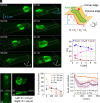
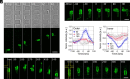
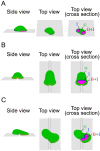
In fast-moving cells such as amoeba and immune cells, dendritic actin filaments are spatiotemporally regulated to shape large-scale plasma membrane protrusions. Despite their importance in migration, as well as in particle and liquid ingestion, how their dynamics are affected by micrometer-scale features of the contact surface is still poorly understood. Here, through quantitative image analysis of Dictyostelium on microfabricated surfaces, we show that there is a distinct mode of topographical guidance directed by the macropinocytic membrane cup. Unlike other topographical guidance known to date that depends on nanometer-scale curvature sensing protein or stress fibers, the macropinocytic membrane cup is driven by the Ras/PI3K/F-actin signaling patch and its dependency on the micrometer-scale topographical features, namely PI3K/F-actin-independent accumulation of Ras-GTP at the convex curved surface, PI3K-dependent patch propagation along the convex edge, and its actomyosin-dependent constriction at the concave edge. Mathematical model simulations demonstrate that the topographically dependent initiation, in combination with the mutually defining patch patterning and the membrane deformation, gives rise to the topographical guidance. Our results suggest that the macropinocytic cup is a self-enclosing structure that can support liquid ingestion by default; however, in the presence of structured surfaces, it is directed to faithfully trace bent and bifurcating ridges for particle ingestion and cell guidance.
Keywords: actin waves; cell migration; contact guidance; macropinocytosis; topography.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
A plasma membrane template for macropinocytic cups.Elife. 2016 Dec 13;5:e20085. doi: 10.7554/eLife.20085. Elife. 2016. PMID: 27960076 Free PMC article.
-
Living on soup: macropinocytic feeding in amoebae.Int J Dev Biol. 2019;63(8-9-10):473-483. doi: 10.1387/ijdb.190220rk. Int J Dev Biol. 2019. PMID: 31840785 Review.
-
Akt and SGK protein kinases are required for efficient feeding by macropinocytosis.J Cell Sci. 2019 Jan 24;132(2):jcs224998. doi: 10.1242/jcs.224998. J Cell Sci. 2019. PMID: 30617109 Free PMC article.
-
Amplification of PIP3 signalling by macropinocytic cups.Biochem J. 2018 Feb 14;475(3):643-648. doi: 10.1042/BCJ20170785. Biochem J. 2018. PMID: 29444849 Free PMC article.
-
Making cups and rings: the 'stalled-wave' model for macropinocytosis.Biochem Soc Trans. 2024 Aug 28;52(4):1785-1794. doi: 10.1042/BST20231426. Biochem Soc Trans. 2024. PMID: 38934501 Free PMC article. Review.
Cited by
-
A minimal physical model for curvotaxis driven by curved protein complexes at the cell's leading edge.Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2306818121. doi: 10.1073/pnas.2306818121. Epub 2024 Mar 15. Proc Natl Acad Sci U S A. 2024. PMID: 38489386 Free PMC article.
-
Competition and synergy of Arp2/3 and formins in nucleating actin waves.Cell Rep. 2024 Jul 23;43(7):114423. doi: 10.1016/j.celrep.2024.114423. Epub 2024 Jul 4. Cell Rep. 2024. PMID: 38968072 Free PMC article.
-
Three-dimensional morphodynamic simulations of macropinocytic cups.iScience. 2021 Oct 1;24(10):103087. doi: 10.1016/j.isci.2021.103087. eCollection 2021 Oct 22. iScience. 2021. PMID: 34755081 Free PMC article.
-
Formation and closure of macropinocytic cups in Dictyostelium.Curr Biol. 2023 Aug 7;33(15):3083-3096.e6. doi: 10.1016/j.cub.2023.06.017. Epub 2023 Jun 27. Curr Biol. 2023. PMID: 37379843 Free PMC article.
-
Nanotopography modulates intracellular excitable systems through cytoskeleton actuation.Proc Natl Acad Sci U S A. 2023 May 9;120(19):e2218906120. doi: 10.1073/pnas.2218906120. Epub 2023 May 1. Proc Natl Acad Sci U S A. 2023. PMID: 37126708 Free PMC article.
References
-
- Reig G., Pulgar E., Concha M. L., Cell migration: From tissue culture to embryos. Development 141, 1999–2013 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

