Intersection of regulatory pathways controlling hemostasis and hemochorial placentation
- PMID: 34876522
- PMCID: PMC8685669
- DOI: 10.1073/pnas.2111267118
Intersection of regulatory pathways controlling hemostasis and hemochorial placentation
Abstract
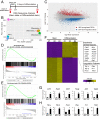
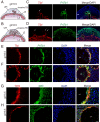
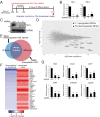
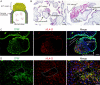
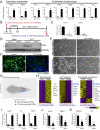
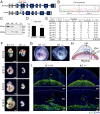
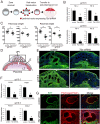
Hemochorial placentation is characterized by the development of trophoblast cells specialized to interact with the uterine vascular bed. We utilized trophoblast stem (TS) cell and mutant rat models to investigate regulatory mechanisms controlling trophoblast cell development. TS cell differentiation was characterized by acquisition of transcript signatures indicative of an endothelial cell-like phenotype, which was highlighted by the expression of anticoagulation factors including tissue factor pathway inhibitor (TFPI). TFPI localized to invasive endovascular trophoblast cells of the rat placentation site. Disruption of TFPI in rat TS cells interfered with development of the endothelial cell-like endovascular trophoblast cell phenotype. Similarly, TFPI was expressed in human invasive/extravillous trophoblast (EVT) cells situated within first-trimester human placental tissues and following differentiation of human TS cells. TFPI was required for human TS cell differentiation to EVT cells. We next investigated the physiological relevance of TFPI at the placentation site. Genome-edited global TFPI loss-of-function rat models revealed critical roles for TFPI in embryonic development, resulting in homogeneous midgestation lethality prohibiting analysis of the role of TFPI as a regulator of the late-gestation wave of intrauterine trophoblast cell invasion. In vivo trophoblast-specific TFPI knockdown was compatible with pregnancy but had profound effects at the uterine-placental interface, including restriction of the depth of intrauterine trophoblast cell invasion while leading to the accumulation of natural killer cells and increased fibrin deposition. Collectively, the experimentation implicates TFPI as a conserved regulator of invasive/EVT cell development, uterine spiral artery remodeling, and hemostasis at the maternal-fetal interface.
Keywords: hemostasis; placenta; trophoblast cell; uterine spiral artery.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2016517118. doi: 10.1073/pnas.2016517118. Proc Natl Acad Sci U S A. 2021. PMID: 33649217 Free PMC article.
-
Modeling Trophoblast Cell-Guided Uterine Spiral Artery Transformation in the Rat.Int J Mol Sci. 2022 Mar 9;23(6):2947. doi: 10.3390/ijms23062947. Int J Mol Sci. 2022. PMID: 35328368 Free PMC article. Review.
-
Conservation at the uterine-placental interface.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2210633119. doi: 10.1073/pnas.2210633119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191208 Free PMC article.
-
FOSL1 is integral to establishing the maternal-fetal interface.Mol Cell Biol. 2011 Dec;31(23):4801-13. doi: 10.1128/MCB.05780-11. Epub 2011 Sep 26. Mol Cell Biol. 2011. PMID: 21947281 Free PMC article.
-
Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling.Placenta. 2021 Sep 15;113:48-56. doi: 10.1016/j.placenta.2021.04.012. Epub 2021 May 3. Placenta. 2021. PMID: 33985793 Free PMC article. Review.
Cited by
-
Tissue Factor Pathway Inhibitors as Potential Targets for Understanding the Pathophysiology of Preeclampsia.Biomedicines. 2023 Apr 22;11(5):1237. doi: 10.3390/biomedicines11051237. Biomedicines. 2023. PMID: 37238908 Free PMC article. Review.
-
The AKT1-FOXO4 axis reciprocally regulates hemochorial placentation.Development. 2023 Jan 15;150(2):dev201095. doi: 10.1242/dev.201095. Epub 2023 Jan 17. Development. 2023. PMID: 36607602 Free PMC article.
-
Sex-differentiated placental methylation and gene expression regulation has implications for neonatal traits and adult diseases.Nat Commun. 2025 May 1;16(1):4004. doi: 10.1038/s41467-025-58128-3. Nat Commun. 2025. PMID: 40312437 Free PMC article.
-
Extravillous trophoblast cell lineage development is associated with active remodeling of the chromatin landscape.Nat Commun. 2023 Aug 10;14(1):4826. doi: 10.1038/s41467-023-40424-5. Nat Commun. 2023. PMID: 37563143 Free PMC article.
-
CORE CONSERVED TRANSCRIPTIONAL REGULATORY NETWORKS DEFINE THE INVASIVE TROPHOBLAST CELL LINEAGE.bioRxiv [Preprint]. 2023 Apr 3:2023.03.30.534962. doi: 10.1101/2023.03.30.534962. bioRxiv. 2023. Update in: Development. 2023 Aug 1;150(15):dev201826. doi: 10.1242/dev.201826. PMID: 37066272 Free PMC article. Updated. Preprint.
References
-
- Kaufmann P., Black S., Huppertz B., Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 69, 1–7 (2003). - PubMed
-
- Pijnenborg R., Vercruysse L., Hanssens M., The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 27, 939–958 (2006). - PubMed
-
- Harris L. K., Aplin J. D., Vascular remodeling and extracellular matrix breakdown in the uterine spiral arteries during pregnancy. Reprod. Sci. 14, 28–34 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

