Runx1 protects against the pathological progression of osteoarthritis
- PMID: 34876557
- PMCID: PMC8651727
- DOI: 10.1038/s41413-021-00173-x
Runx1 protects against the pathological progression of osteoarthritis
Abstract
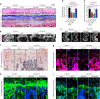
Runt-related transcription factor-1 (Runx1) is required for chondrocyte-to-osteoblast lineage commitment by enhancing both chondrogenesis and osteogenesis during vertebrate development. However, the potential role of Runx1 in joint diseases is not well known. In the current study, we aimed to explore the role of Runx1 in osteoarthritis induced by anterior cruciate ligament transaction (ACLT) surgery. We showed that chondrocyte-specific Runx1 knockout (Runx1f/fCol2a1-Cre) aggravated cartilage destruction by accelerating the loss of proteoglycan and collagen II in early osteoarthritis. Moreover, we observed thinning and ossification of the growth plate, a decrease in chondrocyte proliferative capacity and the loss of bone matrix around the growth plate in late osteoarthritis. We overexpressed Runx1 by adeno-associated virus (AAV) in articular cartilage and identified its protective effect by slowing the destruction of osteoarthritis in cartilage in early osteoarthritis and alleviating the pathological progression of growth plate cartilage in late osteoarthritis. ChIP-seq analysis identified new targets that interacted with Runx1 in cartilage pathology, and we confirmed the direct interactions of these factors with Runx1 by ChIP-qPCR. This study helps us to understand the function of Runx1 in osteoarthritis and provides new clues for targeted osteoarthritis therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

