Underlying mechanisms of glucocorticoid-induced β-cell death and dysfunction: a new role for glycogen synthase kinase 3
- PMID: 34876563
- PMCID: PMC8651641
- DOI: 10.1038/s41419-021-04419-8
Underlying mechanisms of glucocorticoid-induced β-cell death and dysfunction: a new role for glycogen synthase kinase 3
Abstract
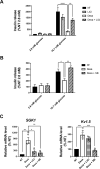
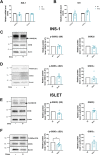
Glucocorticoids (GCs) are widely prescribed for their anti-inflammatory and immunosuppressive properties as a treatment for a variety of diseases. The use of GCs is associated with important side effects, including diabetogenic effects. However, the underlying mechanisms of GC-mediated diabetogenic effects in β-cells are not well understood. In this study we investigated the role of glycogen synthase kinase 3 (GSK3) in the mediation of β-cell death and dysfunction induced by GCs. Using genetic and pharmacological approaches we showed that GSK3 is involved in GC-induced β-cell death and impaired insulin secretion. Further, we unraveled the underlying mechanisms of GC-GSK3 crosstalk. We showed that GSK3 is marginally implicated in the nuclear localization of GC receptor (GR) upon ligand binding. Furthermore, we showed that GSK3 regulates the expression of GR at mRNA and protein levels. Finally, we dissected the proper contribution of each GSK3 isoform and showed that GSK3β isoform is sufficient to mediate the pro-apoptotic effects of GCs in β-cells. Collectively, in this work we identified GSK3 as a viable target to mitigate GC deleterious effects in pancreatic β-cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharm. 2010;10:497–504. - PubMed
-
- Thiele K, Buttgereit F, Huscher D, Zink A, German Collaborative Arthritis Centres. Current use of glucocorticoids in patients with rheumatoid arthritis in Germany. Arthritis Rheum. 2005;53:740–7. - PubMed
-
- Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. ZONDA Trial Investigators. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N. Engl J Med. 2017;376:2448–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

