A 52 weeks dupilumab treatment for moderate to severe atopic dermatitis in Korea: long-term efficacy and safety in real world
- PMID: 34876623
- PMCID: PMC8651808
- DOI: 10.1038/s41598-021-02950-4
A 52 weeks dupilumab treatment for moderate to severe atopic dermatitis in Korea: long-term efficacy and safety in real world
Abstract
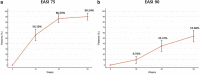
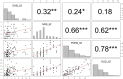
Previously, we have reported short term effectiveness and safety of dupilumab in Korea. In this study, we are trying to report the long-term effectiveness and safety of dupilumab in Korea. Ninety-nine patients with moderate to severe AD were analyzed. They were evaluated using Eczema Area and Severity Index (EASI), Numerical Rating Scale (NRS), Patient Oriented Eczema Measure (POEM), and Dermatology Quality of Life Index (DLQI) at baseline, week 16, 32 and 52. Efficacy outcomes showed higher improvement at 52 weeks compared with 16 weeks; high percentual reductions in EASI (88.1%), peak pruritus NRS (65.6%), POEM (67.2%), and DLQI (69.0%) compared to baseline. Proportion of patients achieving EASI 75 and 90 were 90.2% and 53.7%. POEM and DLQI had high correlation with clinical measured outcomes. In the analysis for the factors affecting achievement of EASI 90, female gender (OR 2.5), eosinophilia (OR 0.2) and elevated LDH (OR 0.07) were significantly associated. Most frequent adverse events included facial erythema (19.2%) and conjunctivitis (17.2%), which were mild/moderate and resolved during treatment. In conclusion, dupilumab treatment for 52 weeks in Korean patients with moderate-to-severe AD confirmed long term effectiveness and safety.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A prospective observational cohort study comparing the treatment effectiveness and safety of ciclosporin, dupilumab and methotrexate in adult and paediatric patients with atopic dermatitis: results from the UK-Irish A-STAR register.Br J Dermatol. 2024 Nov 18;191(6):988-999. doi: 10.1093/bjd/ljae287. Br J Dermatol. 2024. PMID: 39044673
-
Retrospective Study of Dupilumab Treatment for Moderate to Severe Atopic Dermatitis in Korea: Efficacy and Safety of Dupilumab in Real-World Practice.J Clin Med. 2020 Jun 24;9(6):1982. doi: 10.3390/jcm9061982. J Clin Med. 2020. PMID: 32599878 Free PMC article.
-
Efficacy and Safety of Abrocitinib in Patients with Severe and/or Difficult-to-Treat Atopic Dermatitis: A Post Hoc Analysis of the Randomized Phase 3 JADE COMPARE Trial.Am J Clin Dermatol. 2023 Jul;24(4):609-621. doi: 10.1007/s40257-023-00785-5. Epub 2023 May 22. Am J Clin Dermatol. 2023. PMID: 37213005 Free PMC article. Clinical Trial.
-
Dupilumab Improves Clinical Scores in Children and Adolescents With Moderate to Severe Atopic Dermatitis: A Real-World, Single-Center Study.J Allergy Clin Immunol Pract. 2022 Sep;10(9):2378-2385. doi: 10.1016/j.jaip.2022.06.014. Epub 2022 Jun 24. J Allergy Clin Immunol Pract. 2022. PMID: 35753667 Review.
-
Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis.J Am Acad Dermatol. 2021 Jan;84(1):139-147. doi: 10.1016/j.jaad.2020.08.051. Epub 2020 Aug 18. J Am Acad Dermatol. 2021. PMID: 32822798
Cited by
-
Recent updates on systemic treatment of atopic dermatitis.Clin Exp Pediatr. 2024 Nov;67(11):580-588. doi: 10.3345/cep.2024.00339. Epub 2024 Nov 1. Clin Exp Pediatr. 2024. PMID: 39523660 Free PMC article.
-
Burden of Disease and Unmet Needs in the Diagnosis and Management of Atopic Dermatitis in Korea.J Clin Med. 2023 May 29;12(11):3744. doi: 10.3390/jcm12113744. J Clin Med. 2023. PMID: 37297937 Free PMC article. Review.
-
Successful Treatment with Upadacitinib of Patient with Persistent Head and Neck Atopic Dermatitis Refractory to Dupilumab: A Case Report.Indian J Dermatol. 2024 Mar-Apr;69(2):203. doi: 10.4103/ijd.ijd_931_23. Epub 2024 Apr 29. Indian J Dermatol. 2024. PMID: 38841250 Free PMC article. No abstract available.
-
A systematic review of sex and gender differences in treatment outcome of inflammatory skin diseases: Is it time for new guidelines?J Eur Acad Dermatol Venereol. 2025 Mar;39(3):512-528. doi: 10.1111/jdv.20256. Epub 2024 Jul 30. J Eur Acad Dermatol Venereol. 2025. PMID: 39078087 Free PMC article.
-
An unbiased tissue transcriptome analysis identifies potential markers for skin phenotypes and therapeutic responses in atopic dermatitis.Nat Commun. 2025 Jun 2;16(1):4981. doi: 10.1038/s41467-025-59340-x. Nat Commun. 2025. PMID: 40456762 Free PMC article.
References
-
- Silverberg JI, Kantor R. The role of interleukin 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol. Clin. 2017;35:327–334. - PubMed
-
- Wollenberg A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J. Eur. Acad. Dermatol. Venereol. 2018;32:657–682. - PubMed
-
- Wollenberg A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J. Eur. Acad. Dermatol. Venereol. 2018;32:850–878. - PubMed
-
- Ahn J, et al. Emerging systemic therapeutic biologics and small molecules for atopic dermatitis: how to decide which treatment is right for your patients. J. Allergy. Clin. Immunol. Pract. 2021;9:1449–1460. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

