Plasma-derived candidate biomarkers for detection of gallbladder carcinoma
- PMID: 34876625
- PMCID: PMC8651660
- DOI: 10.1038/s41598-021-02923-7
Plasma-derived candidate biomarkers for detection of gallbladder carcinoma
Abstract
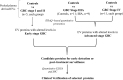
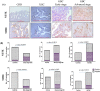
Gallbladder carcinoma (GBC) is a major cancer of the gastrointestinal tract with poor prognosis. Reliable and affordable biomarker-based assays with high sensitivity and specificity for the detection of this cancer are a clinical need. With the aim of studying the potential of the plasma-derived extracellular vesicles (EVs), we carried out quantitative proteomic analysis of the EV proteins, using three types of controls and various stages of the disease, which led to the identification of 86 proteins with altered abundance. These include 29 proteins unique to early stage, 44 unique to the advanced stage and 13 proteins being common to both the stages. Many proteins are functionally relevant to the tumor condition or have been also known to be differentially expressed in GBC tissues. Several of them are also present in the plasma in free state. Clinical verification of three tumor-associated proteins with elevated levels in comparison to all the three control types-5'-nucleotidase isoform 2 (NT5E), aminopeptidase N (ANPEP) and neprilysin (MME) was carried out using individual plasma samples from early or advanced stage GBC. Sensitivity and specificity assessment based on receiver operating characteristic (ROC) analysis indicated a significant association of NT5E and ANPEP with advanced stage GBC and MME with early stage GBC. These and other proteins identified in the study may be potentially useful for developing new diagnostics for GBC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Immunoproteomics approach revealed elevated autoantibody levels against ANXA1 in early stage gallbladder carcinoma.BMC Cancer. 2020 Dec 1;20(1):1175. doi: 10.1186/s12885-020-07676-6. BMC Cancer. 2020. PMID: 33261560 Free PMC article.
-
Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer.World J Gastroenterol. 2014 Apr 14;20(14):4085-92. doi: 10.3748/wjg.v20.i14.4085. World J Gastroenterol. 2014. PMID: 24744600 Free PMC article. Review.
-
Proteomic profiling of cell line-derived extracellular vesicles to identify candidate circulatory markers for detection of gallbladder cancer.Front Oncol. 2022 Nov 23;12:1027914. doi: 10.3389/fonc.2022.1027914. eCollection 2022. Front Oncol. 2022. PMID: 36505879 Free PMC article.
-
Exosomal piRNA profiling revealed unique circulating piRNA signatures of cholangiocarcinoma and gallbladder carcinoma.Acta Biochim Biophys Sin (Shanghai). 2020 May 26;52(5):475-484. doi: 10.1093/abbs/gmaa028. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 32369104 Clinical Trial.
-
Advances and current research status of early diagnosis for gallbladder cancer.Hepatobiliary Pancreat Dis Int. 2025 Jun;24(3):239-251. doi: 10.1016/j.hbpd.2024.09.011. Epub 2024 Oct 4. Hepatobiliary Pancreat Dis Int. 2025. PMID: 39393997 Review.
Cited by
-
Gallbladder cancer: Progress in the Indian subcontinent.World J Clin Oncol. 2024 Jun 24;15(6):695-716. doi: 10.5306/wjco.v15.i6.695. World J Clin Oncol. 2024. PMID: 38946839 Free PMC article. Review.
-
CD13 expression affects glioma patient survival and influences key functions of human glioblastoma cell lines in vitro.BMC Cancer. 2024 Mar 22;24(1):369. doi: 10.1186/s12885-024-12113-z. BMC Cancer. 2024. PMID: 38519889 Free PMC article.
-
Designing of Peptide Based Multi-Epitope Vaccine Construct against Gallbladder Cancer Using Immunoinformatics and Computational Approaches.Vaccines (Basel). 2022 Oct 31;10(11):1850. doi: 10.3390/vaccines10111850. Vaccines (Basel). 2022. PMID: 36366359 Free PMC article.
-
Quantitative tissue proteome profile reveals neutrophil degranulation and remodeling of extracellular matrix proteins in early stage gallbladder cancer.Front Oncol. 2023 Jan 6;12:1046974. doi: 10.3389/fonc.2022.1046974. eCollection 2022. Front Oncol. 2023. PMID: 36686780 Free PMC article.
-
Quantitative proteomic analysis of GnRH agonist treated GBM cell line LN229 revealed regulatory proteins inhibiting cancer cell proliferation.BMC Cancer. 2022 Feb 2;22(1):133. doi: 10.1186/s12885-022-09218-8. BMC Cancer. 2022. PMID: 35109816 Free PMC article.
References
-
- Mahdavifar N, Pakzad R, Ghoncheh M, Gandomani HS, Salehiniya H. Epidemiology, incidence, and mortality of gallbladder cancer and its relation with development in the world. Ann. Trop. Med. Public Health. 2017;10:563–570.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

