Stomach secretes estrogen in response to the blood triglyceride levels
- PMID: 34876651
- PMCID: PMC8651635
- DOI: 10.1038/s42003-021-02901-9
Stomach secretes estrogen in response to the blood triglyceride levels
Abstract
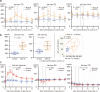
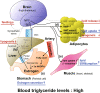
Mammals receive body energy information to maintain energy homeostasis. Ghrelin, insulin, leptin and vagal afferents transmit the status of fasting, blood glucose, body fat, and food intake, respectively. Estrogen also inhibits feeding behavior and lipogenesis, but increases body fat mass. However, how blood triglyceride levels are monitored and the physiological roles of estrogen from the perspective of lipid homeostasis remain unsettled. Here, we show that stomach secretes estrogen in response to the blood triglyceride levels. Estrogen-secreting gastric parietal cells predominantly use fatty acids as an energy source. Blood estrogen levels increase as blood triglyceride levels rise in a stomach-dependent manner. Estrogen levels in stomach tissues increase as blood triglyceride levels rise, and isolated gastric gland epithelium produces estrogen in a fatty acid-dependent manner. We therefore propose that stomach monitors and controls blood triglyceride levels using estrogen, which inhibits feeding behavior and lipogenesis, and promotes triglyceride uptake by adipocytes.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Carbohydrate intake and activation of gastric acid secretion decrease gastric estrogen secretion.Sci Rep. 2025 Mar 28;15(1):10818. doi: 10.1038/s41598-025-95718-z. Sci Rep. 2025. PMID: 40155468 Free PMC article.
-
The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient.Endocrinology. 2004 Nov;145(11):5049-55. doi: 10.1210/en.2004-0493. Epub 2004 Jul 29. Endocrinology. 2004. PMID: 15284203
-
Gastric leptin, but not estrogen and somatostatin, contributes to the elevation of ghrelin mRNA expression level in fasted rats.J Endocrinol. 2008 Mar;196(3):529-38. doi: 10.1677/JOE-07-0300. J Endocrinol. 2008. PMID: 18310448
-
Characteristic features of ghrelin cells in the gastrointestinal tract and the regulation of stomach ghrelin expression and production.World J Gastroenterol. 2008 Nov 7;14(41):6306-11. doi: 10.3748/wjg.14.6306. World J Gastroenterol. 2008. PMID: 19009644 Free PMC article. Review.
-
Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis.Exp Biol Med (Maywood). 2001 Dec;226(11):963-77. doi: 10.1177/153537020122601102. Exp Biol Med (Maywood). 2001. PMID: 11743131 Review.
Cited by
-
Transforming Growth Factor α Evokes Aromatase Expression in Gastric Parietal Cells during Rat Postnatal Development.Int J Mol Sci. 2024 Feb 9;25(4):2119. doi: 10.3390/ijms25042119. Int J Mol Sci. 2024. PMID: 38396796 Free PMC article.
-
Sex-specific effects of dietary restriction on physiological variables in Japanese quails.Ecol Evol. 2024 May 23;14(5):e11405. doi: 10.1002/ece3.11405. eCollection 2024 May. Ecol Evol. 2024. PMID: 38799393 Free PMC article.
-
Tie2-Cre-Induced Inactivation of Non-Nuclear Estrogen Receptor-α Signaling Abrogates Estrogen Protection Against Vascular Injury.JACC Basic Transl Sci. 2022 Nov 16;8(1):55-67. doi: 10.1016/j.jacbts.2022.07.001. eCollection 2023 Jan. JACC Basic Transl Sci. 2022. PMID: 36777173 Free PMC article.
-
Carbohydrate intake and activation of gastric acid secretion decrease gastric estrogen secretion.Sci Rep. 2025 Mar 28;15(1):10818. doi: 10.1038/s41598-025-95718-z. Sci Rep. 2025. PMID: 40155468 Free PMC article.
References
-
- Sato T, et al. Structure, regulation and function of ghrelin. J. Biochem. 2012;151:119–128. - PubMed
-
- Ahima RS, Flier JS. Leptin. Annu. Rev. Physiol. 2000;62:413–437. - PubMed
-
- Maffei, M. & Giordano, A. Leptin, the brain and energy homeostasis: from an apparently simple to a highly complex neuronal system. Rev. Endocr. Metab. Disord.10.1007/s11154-021-09636-2 (2021). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

