Measurement of the Relative Enthalpy of Pure α-AI2O3 (NBS Heat Capacity and Enthalpy Standard Reference Material No. 720) from 273 to 1173 K
- PMID: 34876738
- PMCID: PMC6716031
- DOI: 10.6028/jres.075A.031
Measurement of the Relative Enthalpy of Pure α-AI2O3 (NBS Heat Capacity and Enthalpy Standard Reference Material No. 720) from 273 to 1173 K
Abstract
The relative enthalpy of NBS Standard Reference Material No. 720 (99.98 percent pure, single-crystal α-Al2O3, a calorimetrie heat-capacity standard) was measured over the range 273 to 1173 K by the drop method using a highly precise Bunsen ice calorimeter. Enthalpy data over the same temperature interval were obtained also on the Calorimetry Conference Sample of this substance. These results are believed to be more accurate than similar NBS results on the latter sample published in 1956, and show no significant discontinuity with the NBS data on the same substance that covered the ranges 13 to 380 K and 1173 to 2257 K. The average deviation from the mean for all enthalpy measurements on the SKM 720 sample was 0.017 percent, and the smooth enthalpy values derived from the data were estimated to be accurate to 0.1 percent. The precautions observed in order to minimize measuring errors are described in detail. The data are compared with many sets of the most reliable published data available and new recommended values for the thermodynamic functions of α-Al2O3 are presented for the interval 0 to 1200 K.
Keywords: Alumina; aluminium oxide; corundum; drop calorimetry; enthalpy; heat capacity standard; specific heat; standard reference material; synthetic sapphire; thermodynamic functions.
Figures
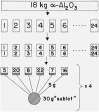
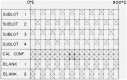
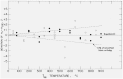
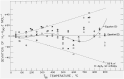
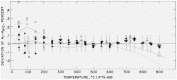
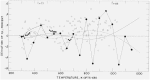
 Martin and Snowdon [40].
Martin and Snowdon [40].

References
-
- Schneider S. J., Pure Appl. Chem. 21, No. 1, 116 (1970).
-
- Furukawa G. T., Douglas T. B., McCoskey R. E., and Ginnings D. C., J. Res. Nat. Bur. Stand. (U.S.). 57, No. 2, 67 (1956) RP2694.
-
- West E. D., and Ishihara S., Advances in Thermophysical Properties at Extreme Temperatures and Pressures, 146–151. The American Society of Mechanical Engineers. N.Y., N.Y. (1965); also in Precision Measurement and Calibration (Heat), Ginnings D. C., Ed., Nat. Bur. Stand. (U.S.), Spec. Publ. 300. Vol. 6, 220–225 (1970).
-
- Verneuil A. V. L., U.S. Patent No. 1.004,505 Sept. 26. 1911.
-
- Douglas T. B., and King E. G., High Temperature Drop Calorimetry, Chapter 8 in Experimental Thermodynamics V.I, (Butterworths, London, 1968); also in Precision Measurement and Calibration (Heat), Ginnings D. C., Ed., Nat. Bur. Stand. (U.S.), Spec. Publ. 300, Vol. 6, 181–219 (1970).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
