Frontiers in antibiotic alternatives for Clostridioides difficile infection
- PMID: 34876784
- PMCID: PMC8611198
- DOI: 10.3748/wjg.v27.i42.7210
Frontiers in antibiotic alternatives for Clostridioides difficile infection
Abstract
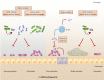
Clostridioides difficile (C. difficile) is a gram-positive, anaerobic spore-forming bacterium and a major cause of antibiotic-associated diarrhea. Humans are naturally resistant to C. difficile infection (CDI) owing to the protection provided by healthy gut microbiota. When the gut microbiota is disturbed, C. difficile can colonize, produce toxins, and manifest clinical symptoms, ranging from asymptomatic diarrhea and colitis to death. Despite the steady-if not rising-prevalence of CDI, it will certainly become more problematic in a world of antibiotic overuse and the post-antibiotic era. C. difficile is naturally resistant to most of the currently used antibiotics as it uses multiple resistance mechanisms. Therefore, current CDI treatment regimens are extremely limited to only a few antibiotics, which include vancomycin, fidaxomicin, and metronidazole. Therefore, one of the main challenges experienced by the scientific community is the development of alternative approaches to control and treat CDI. In this Frontier article, we collectively summarize recent advances in alternative treatment approaches for CDI. Over the past few years, several studies have reported on natural product-derived compounds, drug repurposing, high-throughput library screening, phage therapy, and fecal microbiota transplantation. We also include an update on vaccine development, pre- and pro-biotics for CDI, and toxin antidote approaches. These measures tackle CDI at every stage of disease pathology via multiple mechanisms. We also discuss the gaps and concerns in these developments. The next epidemic of CDI is not a matter of if but a matter of when. Therefore, being well-equipped with a collection of alternative therapeutics is necessary and should be prioritized.
Keywords: Alternative therapy; Bacteriophage; Clostridioides difficile; Drug resistance; Fecal microbiota; Pharmaceutical.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest.
Figures
References
-
- Tullus K, Aronsson B, Marcus S, Möllby R. Intestinal colonization with Clostridium difficile in infants up to 18 months of age. Eur J Clin Microbiol Infect Dis. 1989;8:390–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

