In vitro and in vivo Anti- Toxoplasma Effects of Allium sativum Essential Oil Against Toxoplasma gondii RH Strain
- PMID: 34876824
- PMCID: PMC8643149
- DOI: 10.2147/IDR.S337905
In vitro and in vivo Anti- Toxoplasma Effects of Allium sativum Essential Oil Against Toxoplasma gondii RH Strain
Abstract
Background: Since no effective vaccine has been developed for toxoplasmosis, prophylaxis in seronegative pregnant women and immunocompromised patients with a CD4 <100 cells/μL is highly recommended as an ideal strategy to prevent this disease. This study aimed to assess the chemical composition, in vitro, and in vivo effects of Allium sativum essential oil (ASEO) against Toxoplasma gondii RH strain.
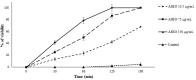
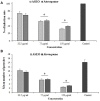
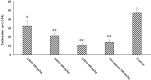
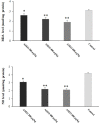
Methods: The in vitro anti-Toxoplasma effects of different concentrations of ASEO (32.5, 75, 150 µg/mL) were measured by MTT assay for 0.5, 1, 2, and 3 h. Male Balb/c mice were orally administrated ASEO at the doses of 200, 400, and 600 µg/kg/day for 14 days. One day after the completion of oral drug administration, the mice in all groups were infected intraperitoneally with 1×104 tachyzoites. They were checked daily and the rate of survival was recorded. The peritoneal fluids of the mice were collected and the mean number of tachyzoites was calculated via a light microscope. The level of liver lipid peroxidation (LPO) and nitric oxide (NO), toxicity effects on the liver and kidney, and the mRNA expression levels of some pro-inflammatory cytokines such as IL-1β and IFN-γ were determined by quantitative real-time PCR.
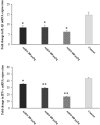
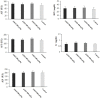
Results: Different concentrations of ASEO showed a significant (p < 0.001) anti-Toxoplasma activity against T. gondii tachyzoites, and the highest efficacy was observed at the concentration of 150 µg/mL. Fourteen days of pre-treatment of infected mice with ASEO at the doses of 200, 400, and 600 µg/kg/day significantly (p < 0.001) decreased the mean number of tachyzoites and mortality rate by the 6th, 7th, and 8th days after infection, respectively. ASEO at the doses of 200, 400, and 600 µg/kg/day significantly (p < 0.05) improved the increase in the LPO and NO. Pre-treatment of mice with different doses of ASEO provoked a considerable (P < 0.001) downregulation of IL-1β and IFN-γ mRNA gene expression levels, but it had no significant toxicity on the serum levels of some liver and kidney enzymes.
Conclusion: The present study demonstrated the considerable prophylactic effects of ASEO that increased the survival rate of mice and reduced the parasite load in them. Our findings also showed that ASEO promotes the innate immune system, pro-inflammatory cytokines, inhibition of hepatic injury, etc. in the mice with acute toxoplasmosis. However, additional investigations are mandatory to clarify the accurate prophylactic and therapeutic anti-Toxoplasma mechanisms of ASEO as well as all its toxicity aspects, especially in clinical settings.
Keywords: RH strain; herbal medicines; prophylaxis; tachyzoites; toxoplasmosis.
© 2021 Alnomasy.
Conflict of interest statement
The author declares no conflict of interest for this work.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

