An Immune-Related lncRNA Signature to Predict the Biochemical Recurrence and Immune Landscape in Prostate Cancer
- PMID: 34876840
- PMCID: PMC8643172
- DOI: 10.2147/IJGM.S336757
An Immune-Related lncRNA Signature to Predict the Biochemical Recurrence and Immune Landscape in Prostate Cancer
Abstract
Purpose: This study aims to construct an immune-related signature to provide comprehensive insights into the immune landscape of prostate cancer, which can predict biochemical recurrence (BCR) and clinical treatment.
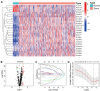
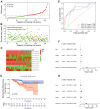
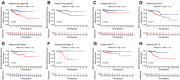
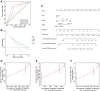
Methods: Based on The Cancer Genome Atlas (TCGA) dataset, a signature constructed by DEirlncRNAs pairs was determined. The receiver operating characteristic curve analysis, Kaplan-Meier analysis, nomogram, and decision curve analysis were used to analyze it. Then, immunophenoscore (IPS), immune cell infiltration, tumor mutation burden (TMB), and immune function were investigated. Finally, we evaluated the role of the signature in medical treatment.
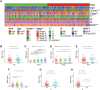
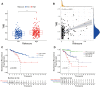
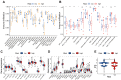
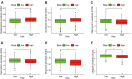
Results: A signature constructed by 10 valid DEirlncRNAs pairs was identified in the training set and validated well in the testing and entire set. The signature was a reliable and independent prognostic indicator to predict the BCR of prostate cancer, which was better than the clinicopathological characteristics. After dividing the patients into low- and high-risk groups by median value, we found that the high-risk group had shorter BCR-free time and higher TMB levels. Furthermore, the high-risk group was negatively associated with plasma B cells and CD+8 T cells. IPS and immune functions, such as immune checkpoints and human leukocyte antigen, were significantly different between the two groups. Low-risk group was more sensitive to endocrine therapy and immunotherapy, while high-risk group was more inclined to targeted drugs. Both groups had their own sensitive chemotherapy.
Conclusion: We established a novel signature to predict BCR and validated its role in the immune landscape of prostate cancer, which could help patients receive personalized medical treatment.
Keywords: TMB; biochemical recurrence; immune landscape; lncRNA; medical treatment; prostate cancer.
© 2021 Zhang and Luo.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
LinkOut - more resources
Full Text Sources

