Regulatory Compliance and Associated Quality of Amoxicillin in Drug Retail Outlets of Southwestern Ethiopia
- PMID: 34876858
- PMCID: PMC8643136
- DOI: 10.2147/DHPS.S337791
Regulatory Compliance and Associated Quality of Amoxicillin in Drug Retail Outlets of Southwestern Ethiopia
Abstract
Background: While the research findings confirm the existence of private drug retail outlets that do not comply with regulatory standards in many low-income countries, there are a lack of reports that evaluate the quality of medicines obtained from these firms. Therefore, the aim of this study was to evaluate the regulatory compliance of the retails and associated quality of amoxicillin in Southwestern Ethiopia.
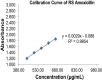
Methodology: Forty-two drug retail outlets in Jimma town were evaluated using an inspection checklist developed by the Ethiopian regulatory authority, and dispensers from these retail outlets were interviewed using the pretested structured questionnaire. The drug outlets were coded and categorized into noncompliant and compliant drug retail outlets. The physicochemical quality of amoxicillin capsules obtained from these retail outlets were evaluated following methods described in the US Pharmacopoeia.
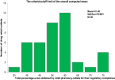
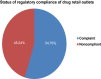
Results: The present study revealed that about 54.76% drug retail outlets were compliant with the regulatory standard. Factors like income of retail outlet, experience of dispenser, and training regarding good storage practice were associated with status of regulatory compliance (p-value <0.05). The identification, dissolution, and assay results indicated that all amoxicillin samples obtained from both noncompliant and compliant drug retail outlets complied with pharmacopoeial specification limit. Besides, the independent unequal variance t-test revealed that there is no significant difference between mean dissolution and assay of API of the amoxicillin samples obtained these drug retail outlets (p-value >0.05).
Conclusion: The regulatory compliance of private drug retail outlets in Jimma town is not satisfactory. Moreover, the laboratory findings revealed that all samples of amoxicillin capsules compiled with pharmacopoeial specifications acceptance for packaging and labeling information, identification, assay, and dissolution. However, despite the fact that assays of the amoxicillin from retail outlets are within the required specification, the assays of amoxicillin obtained from noncompliant retail outlets appears to be slightly degraded, which may potentially demonstrate the impact of noncompliance of the drug retail outlets on the quality of medicines.
Keywords: Ethiopia; amoxicillin; drug retails; quality; regulatory compliance.
© 2021 Aman et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Situational analysis and future directions for medicine retail outlets: compliance with pharmaceutical regulatory standards in Ethiopia.Front Med (Lausanne). 2025 Mar 12;12:1452875. doi: 10.3389/fmed.2025.1452875. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40166068 Free PMC article. Review.
-
Physicochemical Quality Assessment of Antimalarial Medicines: Chloroquine Phosphate and Quinine Sulfate Tablets from Drug Retail Outlets of South-West Ethiopia.Infect Drug Resist. 2020 Feb 27;13:691-701. doi: 10.2147/IDR.S234684. eCollection 2020. Infect Drug Resist. 2020. PMID: 32161477 Free PMC article.
-
Quality Assessment of Selected Essential Antimicrobial Drugs from Drug Retail Outlets of Selected Cities in Eastern Ethiopia.Am J Trop Med Hyg. 2024 Feb 13;110(3):596-608. doi: 10.4269/ajtmh.23-0536. Print 2024 Mar 6. Am J Trop Med Hyg. 2024. PMID: 38350137 Free PMC article.
-
Quality of anthelminthic medicines available in Jimma Ethiopia.Acta Trop. 2018 Jan;177:157-163. doi: 10.1016/j.actatropica.2017.10.006. Epub 2017 Oct 13. Acta Trop. 2018. PMID: 29030043
-
No prescription? No problem: drivers of non-prescribed sale of antibiotics among community drug retail outlets in low and middle income countries: a systematic review of qualitative studies.BMC Public Health. 2021 Jun 3;21(1):1056. doi: 10.1186/s12889-021-11163-3. BMC Public Health. 2021. PMID: 34082726 Free PMC article.
Cited by
-
Effect of tropical climates on the quality of commonly used antibiotics: the protocol for a systematic review and meta-analysis.BMJ Open. 2025 Jan 6;15(1):e090849. doi: 10.1136/bmjopen-2024-090849. BMJ Open. 2025. PMID: 39762102 Free PMC article.
-
In vitro comparative quality evaluation of different brands of Amlodipine Tablets Commercially available in Jimma Town, South-western Ethiopia.PLoS One. 2024 Nov 19;19(11):e0310828. doi: 10.1371/journal.pone.0310828. eCollection 2024. PLoS One. 2024. PMID: 39561126 Free PMC article.
-
Situational analysis and future directions for medicine retail outlets: compliance with pharmaceutical regulatory standards in Ethiopia.Front Med (Lausanne). 2025 Mar 12;12:1452875. doi: 10.3389/fmed.2025.1452875. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40166068 Free PMC article. Review.
References
-
- David L, Mohan J, Thomas L, et al. Ensuring the quality of medicines in resource-limited countries. An operational guide; 2007:11.
-
- World Health Organization. Assessment of medicines regulatory systems in sub-Saharan African countries; 2010:210.
-
- International Pharmaceutical Federation (FIP) and WHO (World Health Organization). Joint FIP/WHO guidelines on good pharmacy practice; 2010:1–19.
-
- Anello E A framework for good governance in the public pharmaceutical sector. Working draft for field testing and revision. Geneva: World Health Organization; 2007.
LinkOut - more resources
Full Text Sources