Quantification of aerosol dispersal from suspected aerosol-generating procedures
- PMID: 34877350
- PMCID: PMC8474485
- DOI: 10.1183/23120541.00206-2021
Quantification of aerosol dispersal from suspected aerosol-generating procedures
Abstract
Background: Oxygen-delivering modalities like humidified high-flow nasal cannula (HFNC) and noninvasive positive-pressure ventilation (NIV) are suspected of generating aerosols that may contribute to transmission of disease such as coronavirus disease 2019. We sought to assess if these modalities lead to increased aerosol dispersal compared to the use of non-humidified low-flow nasal cannula oxygen treatment (LFNC).
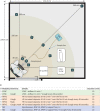
Methods: Aerosol dispersal from 20 healthy volunteers using HFNC, LFNC and NIV oxygen treatment was measured in a controlled chamber. We investigated effects related to coughing and using a surgical face mask in combination with the oxygen delivering modalities. An aerodynamic particle sizer measured aerosol particles (APS3321, 0.3-20 µm) directly in front of the subjects, while a mesh of smaller particle sensors (SPS30, 0.3-10 µm) was distributed in the test chamber.
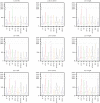
Results: Non-productive coughing led to significant increases in particle dispersal close to the face when using LFNC and HFNC but not when using NIV. HFNC or NIV did not lead to a statistically significant increase in aerosol dispersal compared to LFNC. With non-productive cough in a room without air changes, there was a significant drop in particle levels between 100 cm and 180 cm from the subjects.
Conclusions: Our results indicate that using HFNC and NIV does not lead to increased aerosol dispersal compared to low-flow oxygen treatment, except in rare cases. For a subject with non-productive cough, NIV with double-limb circuit and non-vented mask may be a favourable choice to reduce the risk for aerosol spread.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: R. Strand-Amundsen has nothing to disclose. Conflict of interest: C. Tronstad has nothing to disclose. Conflict of interest: O. Elvebakk has nothing to disclose. Conflict of interest: T. Martinsen has nothing to disclose. Conflict of interest: M. Dybwad has nothing to disclose. Conflict of interest: E. Lingaas has nothing to disclose. Conflict of interest: T.I. Tønnessen has nothing to disclose.
Figures


References
LinkOut - more resources
Full Text Sources
