Contrast-enhanced mammography: what the radiologist needs to know
- PMID: 34877457
- PMCID: PMC8611680
- DOI: 10.1259/bjro.20210034
Contrast-enhanced mammography: what the radiologist needs to know
Abstract
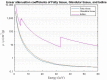
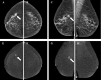
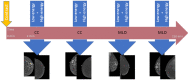
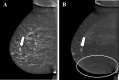
Contrast-enhanced mammography (CEM) is a combination of standard mammography and iodinated contrast material administration. During the last decade, CEM has found its place in breast imaging protocols: after i.v. administration of iodinated contrast material, low-energy and high-energy images are retrieved in one acquisition using a dual-energy technique, and a recombined image is constructed enabling visualisation of areas of contrast uptake. The increased incorporation of CEM into everyday clinical practice is reflected in the installation of dedicated equipment worldwide, the (commercial) availability of systems from different vendors, the number of CEM examinations performed, and the number of scientific articles published on the subject. It follows that ever more radiologists will be confronted with this technique, and thus be required to keep up to date with the latest developments in the field. Most importantly, radiologists must have sufficient knowledge on how to interpret CEM images and be acquainted with common artefacts and pitfalls. This comprehensive review provides a practical overview of CEM technique, including CEM-guided biopsy; reading, interpretation and structured reporting of CEM images, including the accompanying learning curve, CEM artefacts and interpretation pitfalls; indications for CEM; disadvantages of CEM; and future developments.
© 2021 The Authors. Published by the British Institute of Radiology.
Conflict of interest statement
Conflicts of interest: RA received institutional grant and consulting fee from GE Healthcare. JW received institutional grants and speaker’s fees from AGFA, Bayer Healthcare, Bard Medical, GE Healthcare, Optimed, Philips Healthcare, and Siemens Healthineers. ML received several research grant and speaker’s fees from GE Healthcare, Hologic, Bayer, and Guerbet. The other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
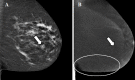
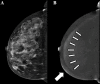
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Medical

