Environmental Thermal Stress Induces Neuronal Cell Death and Developmental Malformations in Reptiles
- PMID: 34877473
- PMCID: PMC8643577
- DOI: 10.1093/iob/obab033
Environmental Thermal Stress Induces Neuronal Cell Death and Developmental Malformations in Reptiles
Abstract
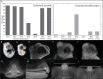
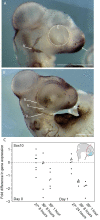
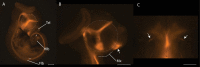
Every stage of organismal life history is being challenged by global warming. Many species are already experiencing temperatures approaching their physiological limits; this is particularly true for ectothermic species, such as lizards. Embryos are markedly sensitive to thermal insult. Here, we demonstrate that temperatures currently experienced in natural nesting areas can modify gene expression levels and induce neural and craniofacial malformations in embryos of the lizard Anolis sagrei. Developmental abnormalities ranged from minor changes in facial structure to significant disruption of anterior face and forebrain. The first several days of postoviposition development are particularly sensitive to this thermal insult. These results raise new concern over the viability of ectothermic species under contemporary climate change. Herein, we propose and test a novel developmental hypothesis that describes the cellular and developmental origins of those malformations: cell death in the developing forebrain and abnormal facial induction due to disrupted Hedgehog signaling. Based on similarities in the embryonic response to thermal stress among distantly related species, we propose that this developmental hypothesis represents a common embryonic response to thermal insult among amniote embryos. Our results emphasize the importance of adopting a broad, multidisciplinary approach that includes both lab and field perspectives when trying to understand the future impacts of anthropogenic change on animal development.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology.
Figures






References
-
- Abayarathna T, Webb JK.. 2020. Effects of incubation temperatures on learning abilities of hatchling velvet geckos. Anim Cogn 23:613–20. - PubMed
-
- Abzhanov A, Tabin CJ.. 2004. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol 273:134–48. - PubMed
-
- Ahlgren SC, Bronner-Fraser M.. 1999. Inhibition of Sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol 9:1304–14. - PubMed
-
- Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341:514–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
