CRISPR-targeted genome editing of human induced pluripotent stem cell-derived hepatocytes for the treatment of Wilson's disease
- PMID: 34877514
- PMCID: PMC8633686
- DOI: 10.1016/j.jhepr.2021.100389
CRISPR-targeted genome editing of human induced pluripotent stem cell-derived hepatocytes for the treatment of Wilson's disease
Abstract
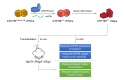
Background & aims: Wilson's disease (WD) is an autosomal recessive disorder of copper metabolism caused by loss-of-function mutations in ATP7B, which encodes a copper-transporting protein. It is characterized by excessive copper deposition in tissues, predominantly in the liver and brain. We sought to investigate whether gene-corrected patient-specific induced pluripotent stem cell (iPSC)-derived hepatocytes (iHeps) could serve as an autologous cell source for cellular transplantation therapy in WD.
Methods: We first compared the in vitro phenotype and cellular function of ATP7B before and after gene correction using CRISPR/Cas9 and single-stranded oligodeoxynucleotides (ssODNs) in iHeps (derived from patients with WD) which were homozygous for the ATP7B R778L mutation (ATP7BR778L/R778L). Next, we evaluated the in vivo therapeutic potential of cellular transplantation of WD gene-corrected iHeps in an immunodeficient WD mouse model (Atp7b -/- / Rag2 -/- / Il2rg -/- ; ARG).
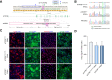
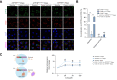
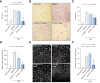
Results: We successfully created iPSCs with heterozygous gene correction carrying 1 allele of the wild-type ATP7B gene (ATP7BWT/-) using CRISPR/Cas9 and ssODNs. Compared with ATP7BR778L/R778L iHeps, gene-corrected ATP7BWT/- iHeps restored i n vitro ATP7B subcellular localization, its subcellular trafficking in response to copper overload and its copper exportation function. Moreover, in vivo cellular transplantation of ATP7BWT/- iHeps into ARG mice via intra-splenic injection significantly attenuated the hepatic manifestations of WD. Liver function improved and liver fibrosis decreased due to reductions in hepatic copper accumulation and consequently copper-induced hepatocyte toxicity.
Conclusions: Our findings demonstrate that gene-corrected patient-specific iPSC-derived iHeps can rescue the in vitro and in vivo disease phenotypes of WD. These proof-of-principle data suggest that iHeps derived from gene-corrected WD iPSCs have potential use as an autologous ex vivo cell source for in vivo therapy of WD as well as other inherited liver disorders.
Lay summary: Gene correction restored ATP7B function in hepatocytes derived from induced pluripotent stem cells that originated from a patient with Wilson's disease. These gene-corrected hepatocytes are potential cell sources for autologous cell therapy in patients with Wilson's disease.
Keywords: AFP, alpha-fetoprotein; ALB, albumin; ATP7B, ATPase copper transporting beta; ATPase copper transporting beta polypeptide (ATP7B); Clustered regularly interspaced palindromic repeats (CRISPR)/Cas9; EB, embryoid body; RFLP, restriction fragment length polymorphism; Single-stranded Oligodeoxynucleotide (ssODN); TGN, trans-Golgi network; WD, Wilson’s disease; Wilson’s disease; cell therapy; gene correction; iHep(s), iPSC-derived hepatocyte(s); iPSC, induced pluripotent stem cell; iPSC-derived hepatocytes (iHeps); induced pluripotent stem cell (iPSC); sgRNA, single guide RNA; ssODN, single-stranded oligodeoxynucleotide.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Similar articles
-
Retinoids rescue ceruloplasmin secretion and alleviate oxidative stress in Wilson's disease-specific hepatocytes.Hum Mol Genet. 2022 Oct 28;31(21):3652-3671. doi: 10.1093/hmg/ddac080. Hum Mol Genet. 2022. PMID: 35388883 Free PMC article.
-
Rescue of ATP7B function in hepatocyte-like cells from Wilson's disease induced pluripotent stem cells using gene therapy or the chaperone drug curcumin.Hum Mol Genet. 2011 Aug 15;20(16):3176-87. doi: 10.1093/hmg/ddr223. Epub 2011 May 18. Hum Mol Genet. 2011. PMID: 21593220
-
Aberrant copper metabolism and hepatic inflammation cause neurological manifestations in a mouse model of Wilson's disease.J Neuroinflammation. 2024 Sep 27;21(1):235. doi: 10.1186/s12974-024-03178-5. J Neuroinflammation. 2024. PMID: 39334421 Free PMC article.
-
Psychiatric Symptoms in Wilson's Disease-Consequence of ATP7B Gene Mutations or Just Coincidence?-Possible Causal Cascades and Molecular Pathways.Int J Mol Sci. 2024 Nov 18;25(22):12354. doi: 10.3390/ijms252212354. Int J Mol Sci. 2024. PMID: 39596417 Free PMC article. Review.
-
Modifying factors and phenotypic diversity in Wilson's disease.Ann N Y Acad Sci. 2014 May;1315:56-63. doi: 10.1111/nyas.12420. Epub 2014 Apr 4. Ann N Y Acad Sci. 2014. PMID: 24702697 Free PMC article. Review.
Cited by
-
Case report: Treatment of Wilson's disease by human amniotic fluid administration.Front Med (Lausanne). 2024 Feb 14;11:1297457. doi: 10.3389/fmed.2024.1297457. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38420355 Free PMC article.
-
Blocking copper transporter protein-dependent drug efflux with albumin-encapsulated Pt(IV) for synergistically enhanced chemo-immunotherapy.J Nanobiotechnology. 2025 Mar 18;23(1):217. doi: 10.1186/s12951-025-03310-4. J Nanobiotechnology. 2025. PMID: 40102840 Free PMC article.
-
Enhancement of hepatic differentiation from induced pluripotent stem cells by suppressing epithelial-mesenchymal transition.Hepatol Commun. 2025 May 16;9(6):e0702. doi: 10.1097/HC9.0000000000000702. eCollection 2025 Jun 1. Hepatol Commun. 2025. PMID: 40377485 Free PMC article.
-
ATP7B gene therapy of autologous reprogrammed hepatocytes alleviates copper accumulation in a mouse model of Wilson's disease.Hepatology. 2022 Oct;76(4):1046-1057. doi: 10.1002/hep.32484. Epub 2022 Apr 13. Hepatology. 2022. PMID: 35340061 Free PMC article.
-
A bioinformatic analysis of gene editing off-target loci altered by common polymorphisms, using 'PopOff'.J R Soc N Z. 2024 May 9;55(6):2440-2463. doi: 10.1080/03036758.2024.2347968. eCollection 2025. J R Soc N Z. 2024. PMID: 40756867 Free PMC article.
References
-
- Huster D. Wilson disease. Best Pract Res Clin Gastroenterol. 2010;24:531–539. - PubMed
-
- Ala A., Walker A.P., Ashkan K., Dooley J.S., Schilsky M.L. Wilson's disease. Lancet. 2007;369:397–408. - PubMed
-
- Fernandes J., Saudubray J.-M., Van den Berghe G., Walter J.H. Springer Science & Business Media; 2006. Inborn metabolic diseases: diagnosis and treatment.
-
- Schilsky M.L. Liver transplantation for Wilson's disease. Ann N Y Acad Sci. 2014;1315:45–49. - PubMed
-
- Filippi C., Dhawan A. Current status of human hepatocyte transplantation and its potential for Wilson's disease. Ann N Y Acad Sci. 2014;1315:50–55. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

