Edible pectin film added with peptides from jackfruit leaves obtained by high-hydrostatic pressure and pepsin hydrolysis
- PMID: 34877530
- PMCID: PMC8633573
- DOI: 10.1016/j.fochx.2021.100170
Edible pectin film added with peptides from jackfruit leaves obtained by high-hydrostatic pressure and pepsin hydrolysis
Abstract
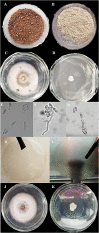
Jackfruit (Artocarpus heterophyllus Lam.) is an evergreen tree that produces a high waste of leaves. This study evaluated the obtention of peptides from jackfruit leaves using pancreatin and pepsin, their antifungal activity, and their effect on pectin films. The protein content was 7.64 ± 0.12 g/100 g of jackfruit fresh leaves. Pancreatin produced a higher yield than pepsin in the obtention of peptides (p ≤ 0.05). However, peptides obtained after 2 h by pepsin hydrolysis (Pep-P) had six essential amino acids and inhibited > 99% of mycelial growth and spore germination of Colletotrichum gloeosporioides. Pectin films with Pep-P showed a slight brown color, lower thickness, water vapor permeability, and moisture content, as well as higher thermal stability and better inhibition properties against C. gloeosporioides than pectin films without Pep-P (p ≤ 0.05). Pectin films added with Pep-P from jackfruit leaf could be a green alternative to anthracnose control in tropical fruits.
Keywords: Acetonitrile (PubChem CID: 6342 HPLC grade ≥99.8%); Amino acid profile; Amino acid standards (AA-S-18); Biocomposite film; Bradford reagent serine standard (PubChem CID: 5951); Colletotrichum gloeosporioides; Glycerol (PubChem CID: 753) sodium hydroxide (NaOH PubChem CID: 14798); Hydrochloric acid (HCl PubChem CID: 313); Jackfruit leaf; L-Norleucine (Nor ≥98% PubChem CID: 21236); Low-methoxyl (LM) pectin (29–33% Food grade E-440); N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA >97% PubChem CID: 2724275); Pancreatin (EC 232-468-9); Pepsin (EC 3.4.23.1); Postharvest protection.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Use of jackfruit leaf (Artocarpus heterophyllus L.) protein hydrolysates as a stabilizer of the nanoemulsions loaded with extract-rich in pentacyclic triterpenes obtained from Coccoloba uvifera L. leaf.Food Chem X. 2021 Oct 7;12:100138. doi: 10.1016/j.fochx.2021.100138. eCollection 2021 Dec 30. Food Chem X. 2021. PMID: 34693274 Free PMC article.
-
Characterization and antifungal activity of jackfruit (Artocarpus heterophyllus Lam.) leaf extract obtained using conventional and emerging technologies.Food Chem. 2020 Nov 15;330:127211. doi: 10.1016/j.foodchem.2020.127211. Epub 2020 Jun 7. Food Chem. 2020. PMID: 32540527
-
Supramolecular structure of jackfruit seed starch and its relationship with digestibility and physicochemical properties.Carbohydr Polym. 2016 Oct 5;150:269-77. doi: 10.1016/j.carbpol.2016.05.030. Epub 2016 May 13. Carbohydr Polym. 2016. PMID: 27312638
-
Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review.Int J Food Sci. 2019 Jan 6;2019:4327183. doi: 10.1155/2019/4327183. eCollection 2019. Int J Food Sci. 2019. PMID: 30723733 Free PMC article. Review.
-
Jackfruit (Artocarpus heterophyllus Lam.) in health and disease: a critical review.Crit Rev Food Sci Nutr. 2023;63(23):6344-6378. doi: 10.1080/10408398.2022.2031094. Epub 2022 Feb 10. Crit Rev Food Sci Nutr. 2023. PMID: 35144492 Review.
Cited by
-
Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds.Molecules. 2021 Dec 23;27(1):60. doi: 10.3390/molecules27010060. Molecules. 2021. PMID: 35011292 Free PMC article. Review.
-
Functionality, physicochemical properties, and applications of chitosan/nano-hydroxyapatite-tea polyphenol films.Food Chem X. 2024 Aug 23;24:101762. doi: 10.1016/j.fochx.2024.101762. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39314538 Free PMC article.
-
Characterization and Applications of the Pectin Extracted from the Peel of Passiflora tripartita var. mollissima.Membranes (Basel). 2023 Sep 16;13(9):797. doi: 10.3390/membranes13090797. Membranes (Basel). 2023. PMID: 37755219 Free PMC article.
-
Guamara and Cocuixtle: Source of Proteases for the Transformation of Shrimp By-Products into Hydrolysates with Potential Application.Biology (Basel). 2023 May 21;12(5):753. doi: 10.3390/biology12050753. Biology (Basel). 2023. PMID: 37237565 Free PMC article.
References
-
- Abdelhedi O., Nasri R., Jridi M., Kchaou H., Nasreddine B., Karbowiak T.…Nasri M. Composite bioactive films based on smooth-hound viscera proteins and gelatin: Physicochemical characterization and antioxidant properties. Food Hydrocolloids. 2018;74:176–186. doi: 10.1016/j.foodhyd.2017.08.006. - DOI
-
- Barbut S., Harper B.A. Dried Ca-alginate films: Effects of glycerol, relative humidity, soy fibers, and carrageenan. LWT - Food Science and Technology. 2019;103:260–265. doi: 10.1016/j.lwt.2019.01.004. - DOI
-
- Calderón-Chiu C., Calderón-Santoyo M., Herman-Lara E., Ragazzo-Sánchez J.A. Jackfruit (Artocarpus heterophyllus Lam) leaf as a new source to obtain protein hydrolysates: Physicochemical characterization, techno-functional properties and antioxidant capacity. Food Hydrocolloids. 2021;112:106319. doi: 10.1016/j.foodhyd.2020.106319. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

