Characteristics of subjective cognitive decline associated with amyloid positivity
- PMID: 34877782
- PMCID: PMC9786747
- DOI: 10.1002/alz.12512
Characteristics of subjective cognitive decline associated with amyloid positivity
Abstract
Introduction: The evidence for characteristics of persons with subjective cognitive decline (SCD) associated with amyloid positivity is limited.
Methods: In 1640 persons with SCD from 20 Amyloid Biomarker Study cohort, we investigated the associations of SCD-specific characteristics (informant confirmation, domain-specific complaints, concerns, feelings of worse performance) demographics, setting, apolipoprotein E gene (APOE) ε4 carriership, and neuropsychiatric symptoms with amyloid positivity.
Results: Between cohorts, amyloid positivity in 70-year-olds varied from 10% to 76%. Only older age, clinical setting, and APOE ε4 carriership showed univariate associations with increased amyloid positivity. After adjusting for these, lower education was also associated with increased amyloid positivity. Only within a research setting, informant-confirmed complaints, memory complaints, attention/concentration complaints, and no depressive symptoms were associated with increased amyloid positivity. Feelings of worse performance were associated with less amyloid positivity at younger ages and more at older ages.
Discussion: Next to age, setting, and APOE ε4 carriership, SCD-specific characteristics may facilitate the identification of amyloid-positive individuals.
Keywords: Alzheimer's disease; amyloid; cerebrospinal fluid; positron emission tomography; subjective cognitive decline.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Willemijn Jansen reports receiving research support from Biogen.
Olin Janssen reports receiving research support from Biogen.
Stephanie Vos reports receiving a personal grant from ZonMw, and research support from Alzheimer Nederland and Stichting Rinsum Ponssen.
Merce Boada reports receiving research support from Grifols and the Instituto de Salud Carlos III (ISCIII) grant; receiving consulting fees; and participating on Data Safety Monitoring Board or Advisory Board from Biogen, Roche, Merck, Zambon, Cortexyme.
Lucilla Parnetti reports receiving funds for clinical trials from INDENA S.P.A., Biogen, EISAI Co., Ltd.; payments were made to University of Perugia, Department of Medicine and Surgery. ‐ From 2019: MIRIADE funded under Marie Skłodowska‐Curie actions by the European Union's Horizon 2020 research and innovation programme. Payments were made to University of Perugia. From 2019: bPRIDE project funded by the European Joint Programming on Neurodegenerative Diseases (JPND, European Union's Horizon 2020 research and innovation programme). Payments were made to University of Perugia; is member of the Editorial Board of the following journals (unpaid): • Neurology • Movement Disorders • Biomolecules • Scientific Reports • Frontiers in Aging Neuroscience • Cells • Journal of Alzheimer Disease • Biomarker Insights • Journal of Integrative Neuroscience Lucilla Parnetti is member of the following societies (unpaid): ‐ Since 2019: Member of the Fresco Network for Parkinson's disease. ‐ Since 2020: Member of the Research Council of Norway, Member of the Icelandic Centre for Research, Coordinator of a Center of Excellence of the Parkinson Foundation; and received Lumipulse kits from Fujirebio and ELISA kits from Euroimmun (2021)
Tormod Fladby reports receiving research support for the present manuscript from European Union (EU)/Joint Programming on Neurodegenerative Diseases (JPND) and The Norwegian Research Council; receiving consulting fees from Biogen and Novo Nordisk; having several planned, issued, and pending patents regarding innate immune amyloid beta clearance and regulation of inflammation; participating on Data Safety Monitoring Board or Advisory Board of Biogen, Novo, and Nordisk; and is leader of the board of Nansen Neuroscience (unpaid).
José Luis Molinuevo is currently a full‐time employee of Lundbeck and earlier has served as a consultant or at advisory boards for the following for‐profit companies, or has given lectures in symposia sponsored by the following for‐profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences, NovoNordisk, Zambón, Cytox, and Nutricia.
Sylvia Villeneuve reports receiving research support from Canadian Institutes of Health Research (CIHR).
Jakub Hort reports research support from Czech Ministry of Health and honoraria for lectures by Schwabe and Lundbeck, as well as travel grant from Czech grant agency and participating in a Data Safety Monitoring Board or Advisory Board from Axon company and having stock options in Alzheon company.
Stéphane Epelbaum reports research support from Fondation Recherche Alzheimer and consulting fees from Biogen and Roche, honoraria for presentation from Eisai, and travel support from Edimark.
Alberto Lleó reports research support from Instituto de Carlos III: Fondo de Investigación Sanitario (PI17:01896; PI20/01330; AC19:00103); payment to the institution Instituto de Carlos III: CIBERNED (COEN); payment to the institution, patent royalties (personal and institutional payments); personal consulting fees from Biogen, Nutricia, Roche, and Fujirebio‐Europe; personal fees from Biogen and Nutricia for lectures or educational programs; and patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued.
Sebastiaan Engelborghs reports participating in Data Safety Monitoring Boards or Advisory Boards of Biogen, Eisai, Icometrix, Novartis, Pfizer, and Roche.
Wiesje M van der Flier reports receiving research funding from ZonMW, NWO, EU‐FP7, EU‐JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health∼Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes‐Strijbis fonds, stichting Equilibrio, Pasman stichting, Biogen MA Inc, Boehringer Ingelheim, Life‐MI, AVID, Roche BV, Fujifilm, and Combinostics. WF holds the Pasman chair. WF has performed contract research for Biogen MA Inc and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape). All funding is paid to her institution; and she is consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc, and is associate editor at
Susan Landau reports receiving support for the present manuscript from NIH U19 AG024904 (PI: Weiner), funds to the institution (UC Berkeley); honorarium for speaking at Hillblom Symposium UC San Francisco 2019; travel fees paid for attending meetings and conferences as member of Scientific Program Committee for the Alzheimer's Association International Conference 2017‐2019; and participating in Advisory Board for KeifeRx.
Julius Popp reports receiving support from Synapsis Foundation Alzheimer Swiss (to institution; Swiss National Research foundation (to institution); Om Pharma Switzerland (to institution), and receiving consulting fees from Om pharma Switzerland (to institution).
Anders Walling reports receiving payment for expert testimony from Lundbeck lecture.
Philip Scheltens reports receiving research support from ZonMW and Alzheimer Nederland and has received consultancy fees (paid to the institution) from AC Immune, Alkermes, Alnylam, Alzheon, Anavex, Biogen, Brainstorm Cell, Cortexyme, Denali, EIP, ImmunoBrain Checkpoint, GemVax, Genentech, Green Valley, Novartis, Novo Nordisk, PeopleBio, Renew LLC, and Roche. He is PI of studies with AC Immune, CogRx, FUJI‐film/Toyama, IONIS, UCB, and Vivoryon. He is a part‐time employee of Life Sciences Partners Amsterdam. He serves on the board of Brain Research Center and New Amsterdam Pharma, and participated on the Data Safety Monitoring Board of Genentech.
Peter J. Snyder is a consultant to AlzeCure Pharma (Sweden).
Chris Rowe reports Cerveau Technologies grant paid to institution Biogen grant paid to institution, Abbvie grant paid to institution, consulting fees from Cerveau Technologies, educational materials preparation payments from Biogen, participating in Cerveau Technologies Scientific Advisory Board, and receiving salary payment from Australian Dementia Network.
Gaël Chételat has received research support from the European Union's Horizon 2020 research and innovation program (grant agreement No. 667696), Inserm, Fondation d'entreprise MMA des Entrepreneurs du Futur, Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Région Normandie, Association France Alzheimer et maladies apparentées, and Fondation Vaincre Alzheimer (all to Inserm); and personal fees from Fondation d'entreprise MMA des Entrepreneurs du Futur. No stock options, patents, or royalties.
Agustin Ruíz receives research support from the Innovative Medicines Initiative 2 Joint Undertaking, which receives support from the European Union's Horizon 2020 research and innovation programme (ADAPTED Grant No. 115975). AR's research is also supported by Instituto de Salud Carlos III (ISCIII) grants PI16/01861 and PI19/01301. Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and funded by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐“Una manera de Hacer Europa) and JPco‐fuND‐2 “Multinational research projects on Personalised Medicine for Neurodegerative Diseases” PREADAPT project, and reports consulting fees from Landsteiner Genmed, Grifols, Araclon biotech and lecture fees from Araclon Biotech; has EU Patent EP21382305.7; and participated on Data Safety Monitoring Board or Advisory Board of Grifols SA and Landsteiner Genmed.
Marta Marquié receives research support from the Instituto de Salud Carlos III (ISCIII) grant PI19/00335 Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and financed by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐“Una manera de Hacer Europa).
PI17/01474 Acción Estratégica en Salud, integrated in the Spanish National R+D+I Plan and financed by ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER‐Una manera de hacer Europa), and reports consulting fees from Grifols, Araclon, Biogen, Roche. Lilly, Schwabe, Cortexyme, Merck and Nutricia.
Henning Boecker received 2 x Promotionsstipendium, University Clinic Bonn, payments were made to institution, and receiving travel support from 2019: Sino‐German Center for research promotion, co‐funded by both DFG and National Science Foundation of China (NSFC) ‐ payments were made to institution (travel reimbursement).
Oliver Peters reports receiving institutional grants from Astra Zeneca, Novartis, Biogen, Genentech, Roche, Eisai, and Fujifilm; and received Consulting fees from Biogen, Roche, Eisai, Abbvie, and Griffols, and received payment or honoraria for lectures from Schwabe, MSD.
Lorena Rami reports research Grant from Spanish government to IDIBAPS (PI Lorena Rami) grant no. PI19/00745.
Alexa Pichet Binette reports Human Amyloid Imaging conference: payment made to me Alzheimer's Association: payments made directly to waive conference fees.
Judes Poirier reports support provided by the Fonds de la Recherche en Santé du Québec and the JL Levesque Foundation and has served at the scientific advisory board of the CIHR and Alzheimer Foundation of France.
Jiri Cerman reports receiving research support from Charles University, and consulting fees from Biogen, as well as participating on Data Safety Monitory Board or Advisory Board of Biogen.
Bruno Dubois received consulting fees from Biogen and research grants for his institution from Roche and Fondation Merck‐Avenir and received Equipment SIMOA paid by Lions Alzheimer.
Daniele Altomare received research support from Institute of Health Carlos III (ISCIII), Spain research grants PI18/00435 and INT19/00016 to DA Department of Health Generalitat de Catalunya PERIS research program SLT006/17/125 to DA; consulting fees for his participation in advisory boards from Fujirebio‐Europe and Roche Diagnostics; speaker honoraria from Fujirebio‐Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A.; and support for attending meetings from Nutricia.
Juan Fortea reports funding from Fondo de Investigaciones Sanitario (FIS), Instituto de Salud Carlos III, to my institution; National Institutes of Health, to institution; Fundació La Marató de TV3, to my institution; Generalitat de Catalunya, to institution; Fundació Catalana Síndrome de Down, to institution; and ‐Fundació Víctor Grífols i Lucas; and has served as a consultant for Novartis and Lundbeck, has received honoraria for lectures from Roche, NovoNordisk, Esteve, and Biogen; has a patent WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease issued; and has served at advisory boards for AC Immune, Zambon, and Lundbeck, as well as institution received support from AC Immune to acquire Quanterix NfL Kits.
Marie Eckerström reports receiving consulting fees from Medavante‐prophase, payment for on‐demand independent reviewer assignments, and payment for lectures from Department of Psychology, University of Gothenburg.
Louisa Thompson reports receiving grant AACSF‐20‐685786 Alzheimer's Association 5/01/2020 – 4/31/2023 payments made to Brown University, and payment or honoraria for lectures from Sage Bionetworks payments.
Victor Villemagne reports receiving consulting fees and payment or honoraria for lectures from IXICO Hospicom ACE Alzheimer's Center.
Rachel Buckley reports research support from NIH‐NIA K99/R00 Pathway to Independence award Alzheimer's Association Research Fellowship and Alzheimer's Association International Conference travel fellowship (2019).
Samantha Burnham reports receiving research support from grants NHMRC GNT1161706, NHMRC GNT1156891, NHMRC GNT1191535, NHMRC GNT2001320, and NHMRC MRFF117 1338.
Marion Delarue reports wages for my work in Chetelat's lab (U1237‐PhIND) at Caen paid by INSERM or University of Caen Normandy (since July 2017); wages for my work in LPCN‐EA7452 at University of Caen Normandy paid by University from IReSP fundings (from January 2021); Punctual Sessions at IFRES (from 2015), paid by "Association Pierre Noal."
Inez Ramakers reports research support from Janssen Pharmaceuticals > to institution and EIT Health > to institution.
Hilkka Soininen reports grants from the Academy of Finland, European Union, and Danone which were paid to institution; as well as personal consultation fee from Novo Nordisk; personal payment for membership of a Data Safety Monitoring Board, of ACImmune. Se also reports having been the head of the board of Muistiliitto, which is the Finnish Alzheimer Association from 2018‐2020 for which no payment was received.
Harald Hampel is an employee of Eisai Inc. and serves as Senior Associate Editor for the journal
Betty M. Tijms is inventor on patent on biological subtypes in AD, No. PCT/NL2020/050216 (owner stichting VUmc).
Frans Verhey reports receiving research support from Profile, EMR Interreg DISTINCT H2020 All to our institution.
Frank Jessen reports receiving research support for the present manuscript from German Center for Neurodegenerative Disorders (DZNE); personal fees for lectures from Lilly and Roche; and personal fees for participating on Data Safety Monitoring Boards or Advisory Boards of Abbvie, AC Immune, Biogen, Böhringer, Danone, Eisai, GE Healthcare, Green Valley, hummingbird, Janssen, MSDOM Pharma, Roche, and Vifer; Executive committee German Psychiatric Association (DGPPN) Vice chair European Alzheimer's Disease Consortium (EADC).
Pieter Jelle Visser reports receiving research support from ZonMW (Redefining AD), from ZonMW (Netherlands Consortium of Dementia Cohorts (NCDC), from Innovative Medicine Initiatives (IMI) (Amypad), from IMI (RADAR‐AD), and from Biogen (Amyloid biomarker study group). All payment were made to institution. He also reports receiving payment for Workshop grant writing organized by Synapsis; patent PCT/NL2020/050216, and is Senior editor Alzheimer Research and Therapy and Biomed Central (BMC) Geriatrics and a Member of the Executive board of European Alzheimer's Disease Cohorts (EADC).
Tomasz Gabryelewicz, Marcel Olde Rikkert, Elena Chipi, Steffen Wolfsgruber, Michael Heneka, Jonas Jarholm, Adrià Tort‐Merino, Pedro Rosa‐Neto, Jiri Cerman, Marc Teichmann, M. Belén Sánchez‐Saudinós, Jarith Ebenau, Cornelia Pocnet, Yvonne Freund‐Levi, Åsa K. Wallin, Magda Tsolaki, Luiza Spiru, and Rik Ossenkoppele have nothing to disclose.
Figures
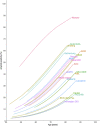
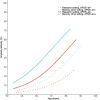


References
-
- Morris JC. Early‐stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:163‐165. - PubMed
-
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130:439‐451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

