The protective gene dose effect of the APOE ε2 allele on gray matter volume in cognitively unimpaired individuals
- PMID: 34877786
- PMCID: PMC9542211
- DOI: 10.1002/alz.12487
The protective gene dose effect of the APOE ε2 allele on gray matter volume in cognitively unimpaired individuals
Abstract
Introduction: Harboring two copies of the apolipoprotein E (APOE) ε2 allele strongly protects against Alzheimer's disease (AD). However, the effect of this genotype on gray matter (GM) volume in cognitively unimpaired individuals has not yet been described.
Methods: Multicenter brain magnetic resonance images (MRIs) from cognitively unimpaired ε2 homozygotes were matched (1:1) against all other APOE genotypes for relevant confounders (n = 223). GM volumes of ε2 genotypic groups were compared to each other and to the reference group (APOE ε3/ε3).
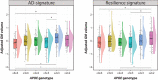
Results: Carrying at least one ε2 allele was associated with larger GM volumes in brain areas typically affected by AD and also in areas associated with cognitive resilience. APOE ε2 homozygotes, but not APOE ε2 heterozygotes, showed larger GM volumes in areas related to successful aging.
Discussion: In addition to the known resistance against amyloid-β deposition, the larger GM volumes in key brain regions may confer APOE ε2 homozygotes additional protection against AD-related cognitive decline.
Keywords: Alzheimer's disease; Alzheimer's disease signature; apolipoprotein E ε2 carrier; brain maintenance; brain morphology; brain reserve; cognitive reserve; magnetic resonance; multi-site; resilience signature.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
JLM is currently a full‐time employee of Lundbeck and priorly has served as a consultant or at advisory boards for the following for‐profit companies, or has given lectures in symposia sponsored by the following for‐profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences. HZ has served on scientific advisory boards for Alector, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, on advisory boards, or on data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. GK is a full‐time employee of Roche Diagnostics GmbH. IS is a full‐time employee and shareholder of Roche Diagnostics International Ltd. The remaining authors declare that they have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

