Development of a model-based clinical trial simulation platform to optimize the design of clinical trials for Duchenne muscular dystrophy
- PMID: 34877803
- PMCID: PMC8923721
- DOI: 10.1002/psp4.12753
Development of a model-based clinical trial simulation platform to optimize the design of clinical trials for Duchenne muscular dystrophy
Abstract
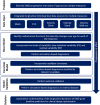
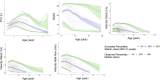
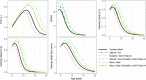
Early clinical trials of therapies to treat Duchenne muscular dystrophy (DMD), a fatal genetic X-linked pediatric disease, have been designed based on the limited understanding of natural disease progression and variability in clinical measures over different stages of the continuum of the disease. The objective was to inform the design of DMD clinical trials by developing a disease progression model-based clinical trial simulation (CTS) platform based on measures commonly used in DMD trials. Data were integrated from past studies through the Duchenne Regulatory Science Consortium founded by the Critical Path Institute (15 clinical trials and studies, 1505 subjects, 27,252 observations). Using a nonlinear mixed-effects modeling approach, longitudinal dynamics of five measures were modeled (NorthStar Ambulatory Assessment, forced vital capacity, and the velocities of the following three timed functional tests: time to stand from supine, time to climb 4 stairs, and 10 meter walk-run time). The models were validated on external data sets and captured longitudinal changes in the five measures well, including both early disease when function improves as a result of growth and development and the decline in function in later stages. The models can be used in the CTS platform to perform trial simulations to optimize the selection of inclusion/exclusion criteria, selection of measures, and other trial parameters. The data sets and models have been reviewed by the US Food and Drug Administration and the European Medicines Agency; have been accepted into the Fit-for-Purpose and Qualification for Novel Methodologies pathways, respectively; and will be submitted for potential endorsement by both agencies.
© 2021 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687‐695. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

