Msl3 promotes germline stem cell differentiation in female Drosophila
- PMID: 34878097
- PMCID: PMC8783043
- DOI: 10.1242/dev.199625
Msl3 promotes germline stem cell differentiation in female Drosophila
Abstract
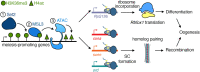
Gamete formation from germline stem cells (GSCs) is essential for sexual reproduction. However, the regulation of GSC differentiation is incompletely understood. Set2, which deposits H3K36me3 modifications, is required for GSC differentiation during Drosophila oogenesis. We discovered that the H3K36me3 reader Male-specific lethal 3 (Msl3) and histone acetyltransferase complex Ada2a-containing (ATAC) cooperate with Set2 to regulate GSC differentiation in female Drosophila. Msl3, acting independently of the rest of the male-specific lethal complex, promotes transcription of genes, including a germline-enriched ribosomal protein S19 paralog RpS19b. RpS19b upregulation is required for translation of RNA-binding Fox protein 1 (Rbfox1), a known meiotic cell cycle entry factor. Thus, Msl3 regulates GSC differentiation by modulating translation of a key factor that promotes transition to an oocyte fate.
Keywords: Differentiation; Meiosis; Msl3; RpS19; Set2 and Rbfox1.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures






References
-
- Anderson, L. K., Royer, S. M., Page, S. L., McKim, K. S., Lai, A., Lilly, M. A. and Hawley, R. S. (2005). Juxtaposition of C(2)M and the transverse filament protein C(3)G within the central region of Drosophila synaptonemal complex. Proc. Natl. Acad. Sci. U.S.A. 102, 4482-4487. 10.1073/pnas.0500172102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

