SARS-CoV-2 Infection Among People Living With HIV Compared With People Without HIV: Survey Results From the MACS-WIHS Combined Cohort Study
- PMID: 34878431
- PMCID: PMC8667184
- DOI: 10.1097/QAI.0000000000002822
SARS-CoV-2 Infection Among People Living With HIV Compared With People Without HIV: Survey Results From the MACS-WIHS Combined Cohort Study
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) symptoms among people living with HIV (PLWH) are not well described.
Setting: Longitudinal survey within the MACS/WIHS Combined Cohort Study (MWCCS) of PLWH compared with similar HIV-seronegative (SN) individuals.
Methods: Telephone-administered survey of MWCCS participants at 13 clinical research sites across the United States addressing COVID-19 symptoms, SARS-CoV-2 testing, and pandemic impact on social distancing and antiretroviral therapy (ART) use. Primary data collection occurred during May (wave 1), June-July (wave 2), and August-September, 2020 (wave 3).
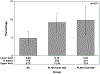
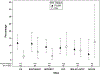
Results: One-third of MWCCS participants were tested for SARS-CoV-2 infection; 10% was tested ≥2 times. Similar proportions of PLWH and SN participants were tested, but SARS-CoV-2 positivity was higher among PLWH than among SN individuals (9.4% vs 4.8%, P = 0.003). Odds of SARS-CoV-2 positivity remained higher among PLWH after adjusting for age, sex, race/ethnicity, and study site (adjusted odds ratio = 2.0, 95% confidence interval = 1.2 to 3.2). SARS-CoV-2 positivity was not associated with CD4 cell counts among PLWH. Among SARS-CoV-2 positive participants, 9% had no symptoms, 7% had 1-2 mild symptoms, and 84% had ≥3 symptoms. Most of the (98%) participants reported physical distancing during all survey waves; self-reported ART adherence among PLWH was not adversely affected during the pandemic compared with the previous year (similar adherence in 89% of participants, improved in 9% of participants, and decreased in 2% of participants).
Conclusions: Despite similar SARS-CoV-2 testing and physical distancing profiles by HIV serostatus among MWCCS participants, PLWH who reported SARS-CoV-2 testing were more likely to have a positive test result. Additional studies are needed to determine whether and why PLWH are at increased risk of SARS-CoV-2 infection.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
T.T.B. has served as a consultant for Merck, Gilead, Janssen, ViiV Healthcare, and Theratechnologies. F.J.P. has served as a consultant and/or speaker for Merck, Gilead, Janssen, ViiV Healthcare, and Theratechnologies. The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- COVID-19 United States Cases by County. Johns Hopkins Coronavirus Resource Center. Accessed January 22, 2021. https://coronavirus.jhu.edu/us-map
-
- CDC. COVIDView, Key Updates for Week 42. Centers for Disease Control and Prevention. Published October 23, 2020. Accessed January 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-repo...
-
- Track Testing Trends. Johns Hopkins Coronavirus Resource Center. Accessed January 22, 2021. https://coronavirus.jhu.edu/testing/tracker
-
- Impact of Opening and Closing Decisions in California, New Cases - Johns Hopkins. Johns Hopkins Coronavirus Resource Center. Accessed January 22, 2021. https://coronavirus.jhu.edu/data/state-timeline
MeSH terms
Grants and funding
- U01 HL146245/HL/NHLBI NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- U01 HL146208/HL/NHLBI NIH HHS/United States
- K23 AI124913/AI/NIAID NIH HHS/United States
- U01 HL146192/HL/NHLBI NIH HHS/United States
- U01 HL146242/HL/NHLBI NIH HHS/United States
- U01 HL146193/HL/NHLBI NIH HHS/United States
- U01 HL146194/HL/NHLBI NIH HHS/United States
- U01 HL146241/HL/NHLBI NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- U01 HL146333/HL/NHLBI NIH HHS/United States
- U01 HL146205/HL/NHLBI NIH HHS/United States
- P30 MH116867/MH/NIMH NIH HHS/United States
- P30 AI073961/AI/NIAID NIH HHS/United States
- U01 HL146201/HL/NHLBI NIH HHS/United States
- R21 AG059505/AG/NIA NIH HHS/United States
- U01 HL146204/HL/NHLBI NIH HHS/United States
- U01 HL146202/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 HL146240/HL/NHLBI NIH HHS/United States
- U01 HL146203/HL/NHLBI NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous