Genital Mucosal Drug Concentrations and anti-HIV Activity in Tenofovir-Based PrEP Products: Intravaginal Ring vs. Oral Administration
- PMID: 34878438
- PMCID: PMC8647693
- DOI: 10.1097/QAI.0000000000002820
Genital Mucosal Drug Concentrations and anti-HIV Activity in Tenofovir-Based PrEP Products: Intravaginal Ring vs. Oral Administration
Abstract
Objective: To describe and compare systemic and local pharmacokinetics (PK) and cervicovaginal (CV) pharmacodynamics (PD) of oral tenofovir disoproxil fumarate (TDF) in combination with emtricitabine (FTC) with tenofovir (TFV) intravaginal ring (IVR).
Design: Phase I, randomized, parallel-group study. Women (n = 22) used TDF/FTC oral tablets daily or TFV IVR continuously and were assessed at baseline and 14 days.
Methods: TFV and FTC concentrations were measured in plasma, CV fluid (CVF), and CV tissue. TFV-diphosphate and FTC-triphosphate were assessed in CV tissue. In vitro PD antiviral activities of TFV and FTC (using in vivo concentration ranges) were modeled in the CVF and by infecting CV tissue explants ex vivo with HIV-1BaL.
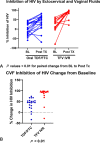
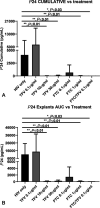
Results: Adverse events (AEs) were more common with oral TDF/FTC use (P < 0.01). The median CVF TFV concentrations were 106 ng/mL after use of TFV IVR vs. 102 ng/mL for TDF/FTC. The median TFV and TFV-diphosphate concentrations in CV tissue were >100-fold higher among IVR users. The median CVF FTC concentrations were 103 ng/mL. FTC and FTC-triphosphate were detected in all CV tissues from TDF/FTC users. HIV inhibitory activity of CVF increased significantly with treatment in both cohorts (P < 0.01) but was higher in TFV IVR users (P < 0.01). In vitro inhibition of tissue infection with ex vivo administration of TFV and FTC was dose dependent, with maximal efficacy achieved with 10 µg/mL TFV, 1 µg/mL FTC, and 0.1 µg/mL of TFV and FTC combined.
Conclusions: Both products were safe and increased mucosal HIV inhibitory activity. In addition to systemic protection, oral TDF/FTC displays a PK/PD profile compatible with CV mucosal antiviral activity. TFV IVR resulted in fewer AEs, lower TFV plasma concentrations, higher CVF and tissue TFV and TFV-DP concentrations, and greater anti-HIV activity in CVF.
Trial registration: ClinicalTrials.gov NCT02722343 NCT02904369.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- United Nations. Global AIDS Update 2018Miles to Go: Closing Gaps, Breaking Barriers, Righting Injustices. Available at: http://wwwunaidsorg/sites/default/files/media_asset/miles-to-go_enpdf. 2018. Accessed January 10, 2021.
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. - PubMed
-
- Riddell Jt, Amico KR, Mayer KH. HIV preexposure prophylaxis: a Review. JAMA. 2018;319:1261–1268. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous