Release of Molecular Cargo from Polymer Systems by Mechanochemistry
- PMID: 34878679
- PMCID: PMC9306765
- DOI: 10.1002/chem.202103860
Release of Molecular Cargo from Polymer Systems by Mechanochemistry
Abstract
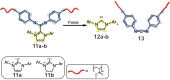
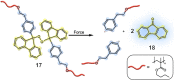
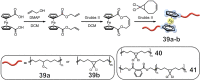
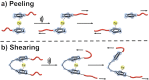
The design and manipulation of (multi)functional materials at the nanoscale holds the promise of fuelling tomorrow's major technological advances. In the realm of macromolecular nanosystems, the incorporation of force-responsive groups, so called mechanophores, has resulted in unprecedented access to responsive behaviours and enabled sophisticated functions of the resulting structures and advanced materials. Among the diverse force-activated motifs, the on-demand release or activation of compounds, such as catalysts, drugs, or monomers for self-healing, are sought-after since they enable triggering pristine small molecule function from macromolecular frameworks. Here, we highlight examples of molecular cargo release systems from polymer-based architectures in solution by means of sonochemical activation by ultrasound (ultrasound-induced mechanochemistry). Important design concepts of these advanced materials are discussed, as well as their syntheses and applications.
Keywords: drug release; mechanochemistry; polymers; sonochemistry; ultrasound.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



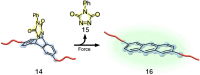

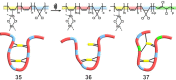





References
-
- None
-
- Panja S., Adams D. J., Chem. Soc. Rev. 2021, 50, 5165–5200; - PubMed
-
- McCracken J. M., Donovan B. R., White T. J., Adv. Mater. 2020, 32, 19906564; - PubMed
-
- Kocak G., Tuncer C., Bütün V., Polym. Chem. 2017, 8, 144–176;
-
- Segarra-Maset M. D., Nebot V. J., Miravet J. F., Escuder B., Chem. Soc. Rev. 2013, 42, 7086–7098; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

