Cytoskeletal protein degradation in brain death donor kidneys associates with adverse posttransplant outcomes
- PMID: 34878723
- PMCID: PMC9305475
- DOI: 10.1111/ajt.16912
Cytoskeletal protein degradation in brain death donor kidneys associates with adverse posttransplant outcomes
Abstract
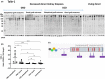
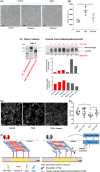
In brain death, cerebral injury contributes to systemic biological dysregulation, causing significant cellular stress in donor kidneys adversely impacting the quality of grafts. Here, we hypothesized that donation after brain death (DBD) kidneys undergo proteolytic processes that may deem grafts susceptible to posttransplant dysfunction. Using mass spectrometry and immunoblotting, we mapped degradation profiles of cytoskeletal proteins in deceased and living donor kidney biopsies. We found that key cytoskeletal proteins in DBD kidneys were proteolytically cleaved, generating peptide fragments, predominantly in grafts with suboptimal posttransplant function. Interestingly, α-actinin-4 and talin-1 proteolytic fragments were detected in brain death but not in circulatory death or living donor kidneys with similar donor characteristics. As talin-1 is a specific proteolytic target of calpain-1, we investigated a potential trigger of calpain activation and talin-1 degradation using human ex vivo precision-cut kidney slices and in vitro podocytes. Notably, we showed that activation of calpain-1 by transforming growth factor-β generated proteolytic fragments of talin-1 that matched the degradation fragments detected in DBD preimplantation kidneys, also causing dysregulation of the actin cytoskeleton in human podocytes; events that were reversed by calpain-1 inhibition. Our data provide initial evidence that brain death donor kidneys are more susceptible to cytoskeletal protein degradation. Correlation to posttransplant outcomes may be established by future studies.
Keywords: QUOD biobank; basic (laboratory) research/science; cellular biology; donors and donation: donation after brain death (DBD); glomerular biology and disease; kidney (allograft) function/dysfunction; kidney failure/injury; kidney transplantation/nephrology; organ transplantation in general; proteomics; translational research/science.
© 2022 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

