Mechanistic Basis for Understanding the Dual Activities of the Bifunctional Azotobacter vinelandii Mannuronan C-5-Epimerase and Alginate Lyase AlgE7
- PMID: 34878812
- PMCID: PMC8824271
- DOI: 10.1128/AEM.01836-21
Mechanistic Basis for Understanding the Dual Activities of the Bifunctional Azotobacter vinelandii Mannuronan C-5-Epimerase and Alginate Lyase AlgE7
Abstract
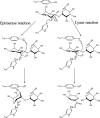
The structure and functional properties of alginates are dictated by the monomer composition and molecular weight distribution. Mannuronan C-5-epimerases determine the monomer composition by catalyzing the epimerization of β-d-mannuronic acid (M) residues into α-l-guluronic acid (G) residues. The molecular weight is affected by alginate lyases, which catalyze a β-elimination mechanism that cleaves alginate chains. The reaction mechanisms for the epimerization and lyase reactions are similar, and some enzymes can perform both reactions. These dualistic enzymes share high sequence identity with mannuronan C-5-epimerases without lyase activity. The mechanism behind their activity and the amino acid residues responsible for it are still unknown. We investigate mechanistic determinants involved in the bifunctional epimerase and lyase activity of AlgE7 from Azotobacter vinelandii. Based on sequence analyses, a range of AlgE7 variants were constructed and subjected to activity assays and product characterization by nuclear magnetic resonance (NMR) spectroscopy. Our results show that calcium promotes lyase activity, whereas NaCl reduces the lyase activity of AlgE7. By using defined polymannuronan (polyM) and polyalternating alginate (polyMG) substrates, the preferred cleavage sites of AlgE7 were found to be M|XM and G|XM, where X can be either M or G. From the study of AlgE7 mutants, R148 was identified as an important residue for the lyase activity, and the point mutant R148G resulted in an enzyme with only epimerase activity. Based on the results obtained in the present study, we suggest a unified catalytic reaction mechanism for both epimerase and lyase activities where H154 functions as the catalytic base and Y149 functions as the catalytic acid. IMPORTANCE Postharvest valorization and upgrading of algal constituents are promising strategies in the development of a sustainable bioeconomy based on algal biomass. In this respect, alginate epimerases and lyases are valuable enzymes for tailoring the functional properties of alginate, a polysaccharide extracted from brown seaweed with numerous applications in food, medicine, and material industries. By providing a better understanding of the catalytic mechanism and of how the two enzyme actions can be altered by changes in reaction conditions, this study opens further applications of bacterial epimerases and lyases in the enzymatic tailoring of alginate polymers.
Keywords: alginate; alginate C-5-epimerase; alginate lyase; enzyme mechanism; multifunctional enzyme; nuclear magnetic resonance (NMR); site‐directed mutagenesis; time-resolved NMR.
Conflict of interest statement
The authors declare no conflict of interest.
We declare that we have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
The catalytic activities of the bifunctional Azotobacter vinelandii mannuronan C-5-epimerase and alginate lyase AlgE7 probably originate from the same active site in the enzyme.J Biol Chem. 2001 Aug 24;276(34):31542-50. doi: 10.1074/jbc.M102562200. Epub 2001 Jun 4. J Biol Chem. 2001. PMID: 11390391
-
Characterization of three new Azotobacter vinelandii alginate lyases, one of which is involved in cyst germination.J Bacteriol. 2009 Aug;191(15):4845-53. doi: 10.1128/JB.00455-09. Epub 2009 May 29. J Bacteriol. 2009. PMID: 19482920 Free PMC article.
-
Characterization of a novel bifunctional mannuronan C-5 epimerase and alginate lyase from Pseudomonas mendocina. sp. DICP-70.Int J Biol Macromol. 2020 May 1;150:662-670. doi: 10.1016/j.ijbiomac.2020.02.126. Epub 2020 Feb 13. Int J Biol Macromol. 2020. PMID: 32061850
-
Mannuronate C-5 epimerases and their use in alginate modification.Essays Biochem. 2023 Apr 18;67(3):615-627. doi: 10.1042/EBC20220151. Essays Biochem. 2023. PMID: 36876890 Review.
-
Hexuronyl C5-epimerases in alginate and glycosaminoglycan biosynthesis.Biochimie. 2001 Aug;83(8):819-30. doi: 10.1016/s0300-9084(01)01313-x. Biochimie. 2001. PMID: 11530215 Review.
Cited by
-
Computational modeling of the molecular basis for the calcium-dependence of the mannuronan C-5 epimerase AvAlgE6 from Azotobacter vinelandii.Comput Struct Biotechnol J. 2023 Mar 15;21:2188-2196. doi: 10.1016/j.csbj.2023.03.021. eCollection 2023. Comput Struct Biotechnol J. 2023. PMID: 37013001 Free PMC article.
-
Diatom heterotrophy on brown algal polysaccharides emerged through horizontal gene transfer, gene duplication, and neofunctionalization.PLoS Biol. 2025 Apr 1;23(4):e3003038. doi: 10.1371/journal.pbio.3003038. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40168346 Free PMC article.
-
Mode of Action of AlgE1: A Modular Mannuronate C-5 Epimerase.Biochemistry. 2025 Jul 15;64(14):3030-3044. doi: 10.1021/acs.biochem.5c00156. Epub 2025 Jun 23. Biochemistry. 2025. PMID: 40549830 Free PMC article.
References
-
- Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K. 2016. Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016:e7697031. 10.1155/2016/7697031. - DOI
-
- Moradali MF, Ghods S, Rehm BHA. 2018. Alginate biosynthesis and biotechnological production, p 1–25. In Rehm BHA, Moradali MF (ed), Alginates and their biomedical applications. Springer series in biomaterials science and engineering, vol 11. Springer, Singapore. 10.1007/978-981-10-6910-9_1. - DOI
-
- Draget KI, Skjåk Bræk G, Smidsrød O. 1994. Alginic acid gels: the effect of alginate chemical composition and molecular weight. Carbohydr Polym 25:31–38. 10.1016/0144-8617(94)90159-7. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

