Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence
- PMID: 34878825
- PMCID: PMC9869449
- DOI: 10.1126/scitranslmed.abh1962
Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence
Erratum in
-
Erratum for the Research Article "Combining a CAR and a chimeric costimulatory receptor enhances T cell sensitivity to low antigen density and promotes persistence" by A. Katsarou et al.Sci Transl Med. 2025 Jul 23;17(808):eaea1242. doi: 10.1126/scitranslmed.aea1242. Epub 2025 Jul 23. Sci Transl Med. 2025. PMID: 40700522 No abstract available.
Abstract
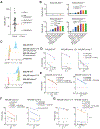
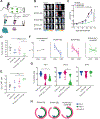
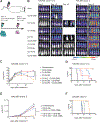
Despite the high remission rates achieved using T cells bearing a chimeric antigen receptor (CAR) against hematogical malignancies, there is still a considerable proportion of patients who eventually experience tumor relapse. Clinical studies have established that mechanisms of treatment failure include the down-regulation of target antigen expression and the limited persistence of effective CAR T cells. We hypothesized that dual targeting mediated by a CAR and a chimeric costimulatory receptor (CCR) could simultaneously enhance T cell cytotoxicity and improve durability. Concomitant high-affinity engagement of a CD38-binding CCR enhanced the cytotoxicity of BCMA-CAR and CD19-CAR T cells by increasing their functional binding avidity. In comparison to second-generation BCMA-CAR or CD19-CAR T cells, double-targeted CAR + CD38-CCR T cells exhibited increased sensitivity to recognize and lyse tumor variants of multiple myeloma and acute lymphoblastic leukemia with low antigen density in vitro. In addition, complimentary costimulation by 4-1BB and CD28 endodomains provided by the CAR and CCR combination conferred increased cytokine secretion and expansion and improved persistence in vivo. The cumulatively improved properties of CAR + CCR T cells enabled the in vivo eradication of antigen-low tumor clones, which were otherwise resistant to treatment with conventional CAR T cells. Therefore, multiplexing targeting and costimulation through the combination of a CAR and a CCR is a powerful strategy to improve the clinical outcomes of CAR T cells by enhancing cytotoxic efficacy and persistence, thus preventing relapses of tumor clones with low target antigen density.
Conflict of interest statement
Figures




References
-
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA, Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378, 439–448 (2018). - PMC - PubMed
-
- Majzner RG, Mackall CL, Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med 25, 1341–1355 (2019). - PubMed
-
- Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL, CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24, 20–28 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

