The 3-phosphoinositide-dependent protein kinase 1 is an essential upstream activator of protein kinase A in malaria parasites
- PMID: 34879056
- PMCID: PMC8687544
- DOI: 10.1371/journal.pbio.3001483
The 3-phosphoinositide-dependent protein kinase 1 is an essential upstream activator of protein kinase A in malaria parasites
Abstract
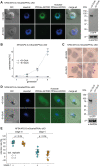
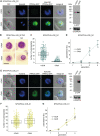
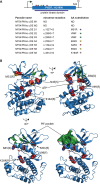
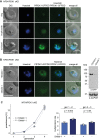
Cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) signalling is essential for the proliferation of Plasmodium falciparum malaria blood stage parasites. The mechanisms regulating the activity of the catalytic subunit PfPKAc, however, are only partially understood, and PfPKAc function has not been investigated in gametocytes, the sexual blood stage forms that are essential for malaria transmission. By studying a conditional PfPKAc knockdown (cKD) mutant, we confirm the essential role for PfPKAc in erythrocyte invasion by merozoites and show that PfPKAc is involved in regulating gametocyte deformability. We furthermore demonstrate that overexpression of PfPKAc is lethal and kills parasites at the early phase of schizogony. Strikingly, whole genome sequencing (WGS) of parasite mutants selected to tolerate increased PfPKAc expression levels identified missense mutations exclusively in the gene encoding the parasite orthologue of 3-phosphoinositide-dependent protein kinase-1 (PfPDK1). Using targeted mutagenesis, we demonstrate that PfPDK1 is required to activate PfPKAc and that T189 in the PfPKAc activation loop is the crucial target residue in this process. In summary, our results corroborate the importance of tight regulation of PfPKA signalling for parasite survival and imply that PfPDK1 acts as a crucial upstream regulator in this pathway and potential new drug target.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Protein Kinase A Is Essential for Invasion of Plasmodium falciparum into Human Erythrocytes.mBio. 2019 Oct 8;10(5):e01972-19. doi: 10.1128/mBio.01972-19. mBio. 2019. PMID: 31594816 Free PMC article.
-
cAMP signalling and its role in host cell invasion by malaria parasites.Curr Opin Microbiol. 2020 Dec;58:69-74. doi: 10.1016/j.mib.2020.09.003. Epub 2020 Oct 5. Curr Opin Microbiol. 2020. PMID: 33032143 Review.
-
Expression and biochemical characterization of the Plasmodium falciparum protein kinase A catalytic subunit.Parasitol Res. 2009 Jun;104(6):1299-305. doi: 10.1007/s00436-008-1327-3. Epub 2009 Jan 22. Parasitol Res. 2009. PMID: 19159956
-
Phenotypic and transcriptomic analyses of Plasmodium falciparum protein kinase A catalytic subunit inhibition.Parasitol Res. 2009 Nov;105(6):1691-9. doi: 10.1007/s00436-009-1615-6. Epub 2009 Sep 25. Parasitol Res. 2009. PMID: 19779742
-
cAMP-dependent protein kinase from Plasmodium falciparum: an update.Parasitology. 2011 Jan;138(1):1-25. doi: 10.1017/S003118201000096X. Epub 2010 Jul 21. Parasitology. 2011. PMID: 20663247 Review.
Cited by
-
Proteomic approaches for protein kinase substrate identification in Apicomplexa.Mol Biochem Parasitol. 2024 Sep;259:111633. doi: 10.1016/j.molbiopara.2024.111633. Epub 2024 May 29. Mol Biochem Parasitol. 2024. PMID: 38821187 Review.
-
SPARK regulates AGC kinases central to the Toxoplasma gondii asexual cycle.Elife. 2024 Aug 13;13:RP93877. doi: 10.7554/eLife.93877. Elife. 2024. PMID: 39136687 Free PMC article.
-
Activation loop phosphorylation and cGMP saturation of PKG regulate egress of malaria parasites.PLoS Pathog. 2024 Jun 27;20(6):e1012360. doi: 10.1371/journal.ppat.1012360. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38935780 Free PMC article.
-
Plasmodium falciparum GAP40 Plays an Essential Role in Merozoite Invasion and Gametocytogenesis.Microbiol Spectr. 2023 Jun 15;11(3):e0143423. doi: 10.1128/spectrum.01434-23. Epub 2023 May 30. Microbiol Spectr. 2023. PMID: 37249423 Free PMC article.
-
SPARK regulates AGC kinases central to the Toxoplasma gondii asexual cycle.bioRxiv [Preprint]. 2024 May 2:2023.10.30.564746. doi: 10.1101/2023.10.30.564746. bioRxiv. 2024. Update in: Elife. 2024 Aug 13;13:RP93877. doi: 10.7554/eLife.93877. PMID: 37961644 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

