The neutrophil protein CD177 is a novel PDPN receptor that regulates human cancer-associated fibroblast physiology
- PMID: 34879110
- PMCID: PMC8654239
- DOI: 10.1371/journal.pone.0260800
The neutrophil protein CD177 is a novel PDPN receptor that regulates human cancer-associated fibroblast physiology
Abstract
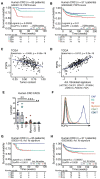
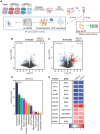
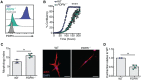
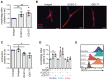
The cancer-associated fibroblast (CAF) marker podoplanin (PDPN) is generally correlated with poor clinical outcomes in cancer patients and thus represents a promising therapeutic target. Despite its biomedical relevance, basic aspects of PDPN biology such as its cellular functions and cell surface ligands remain poorly uncharacterized, thus challenging drug development. Here, we utilize a high throughput platform to elucidate the PDPN cell surface interactome, and uncover the neutrophil protein CD177 as a new binding partner. Quantitative proteomics analysis of the CAF phosphoproteome reveals a role for PDPN in cell signaling, growth and actomyosin contractility, among other processes. Moreover, cellular assays demonstrate that CD177 is a functional antagonist, recapitulating the phenotype observed in PDPN-deficient CAFs. In sum, starting from the unbiased elucidation of the PDPN co-receptome, our work provides insights into PDPN functions and reveals the PDPN/CD177 axis as a possible modulator of fibroblast physiology in the tumor microenvironment.
Conflict of interest statement
Y.S., S.G., V.C.P., C.C., J.R.L., and S.J.T. are Genentech employees and own shares in the Genentech/Roche group. J.L.A, S.K., B.H., E.V., S.M.P., A.A.P., L.G. and N.M.M. were employees of Roche when the data in this paper was generated.
Figures

Similar articles
-
Roles of Podoplanin in Malignant Progression of Tumor.Cells. 2022 Feb 7;11(3):575. doi: 10.3390/cells11030575. Cells. 2022. PMID: 35159384 Free PMC article. Review.
-
Podoplanin Expression in Early-Stage Colorectal Cancer-Associated Fibroblasts and Its Utility as a Diagnostic Marker for Colorectal Lesions.Cells. 2024 Oct 11;13(20):1682. doi: 10.3390/cells13201682. Cells. 2024. PMID: 39451200 Free PMC article.
-
Podoplanin, α-smooth muscle actin or S100A4 expressing cancer-associated fibroblasts are associated with different prognosis in colorectal cancers.J Korean Med Sci. 2013 Sep;28(9):1293-301. doi: 10.3346/jkms.2013.28.9.1293. Epub 2013 Aug 28. J Korean Med Sci. 2013. PMID: 24015033 Free PMC article.
-
Expression of Podoplanin in Mammary Cancers in Female Dogs.In Vivo. 2020 Jan-Feb;34(1):213-223. doi: 10.21873/invivo.11763. In Vivo. 2020. PMID: 31882481 Free PMC article.
-
Neutrophil-specific antigen HNA-2a (NB1, CD177): serology, biochemistry, and molecular biology.Vox Sang. 2002 Aug;83 Suppl 1:359-61. doi: 10.1111/j.1423-0410.2002.tb05334.x. Vox Sang. 2002. PMID: 12617169 Review. No abstract available.
Cited by
-
Roles of Podoplanin in Malignant Progression of Tumor.Cells. 2022 Feb 7;11(3):575. doi: 10.3390/cells11030575. Cells. 2022. PMID: 35159384 Free PMC article. Review.
-
Cell surface protein-protein interaction profiling for biological network analysis and novel target discovery.Life Med. 2024 Aug 29;3(4):lnae031. doi: 10.1093/lifemedi/lnae031. eCollection 2024 Aug. Life Med. 2024. PMID: 39872863 Free PMC article. Review.
-
Tumor Suppression by Anti-Fibroblast Activation Protein Near-Infrared Photoimmunotherapy Targeting Cancer-Associated Fibroblasts.Cancers (Basel). 2024 Jan 20;16(2):449. doi: 10.3390/cancers16020449. Cancers (Basel). 2024. PMID: 38275890 Free PMC article.
-
Modulation of the antitumor immune response by cancer-associated fibroblasts: mechanisms and targeting strategies to hamper their immunosuppressive functions.Explor Target Antitumor Ther. 2022;3(5):598-629. doi: 10.37349/etat.2022.00103. Epub 2022 Oct 27. Explor Target Antitumor Ther. 2022. PMID: 36338519 Free PMC article. Review.
-
Dual role of CD177 + neutrophils in inflammatory bowel disease: a review.J Transl Med. 2024 Sep 2;22(1):813. doi: 10.1186/s12967-024-05539-3. J Transl Med. 2024. PMID: 39223577 Free PMC article. Review.
References
-
- Cremasco V, Astarita JL, Grauel AL, Keerthivasan S, MacIsaac K, Woodruff MC, et al.. FAP Delineates Heterogeneous and Functionally Divergent Stromal Cells in Immune-Excluded Breast Tumors. Cancer Immunol Res. 2018;6(12):1472–85. Epub 2018/09/30. doi: 10.1158/2326-6066.CIR-18-0098 . - DOI - PMC - PubMed
-
- Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al.. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020;10(2):232–53. Epub 2019/11/09. doi: 10.1158/2159-8290.CD-19-0644 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

