Effect of Use of a Bougie vs Endotracheal Tube With Stylet on Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial
- PMID: 34879143
- PMCID: PMC8655668
- DOI: 10.1001/jama.2021.22002
Effect of Use of a Bougie vs Endotracheal Tube With Stylet on Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation: A Randomized Clinical Trial
Abstract
Importance: For critically ill adults undergoing emergency tracheal intubation, failure to intubate the trachea on the first attempt occurs in up to 20% of cases and is associated with severe hypoxemia and cardiac arrest. Whether using a tracheal tube introducer ("bougie") increases the likelihood of successful intubation compared with using an endotracheal tube with stylet remains uncertain.
Objective: To determine the effect of use of a bougie vs an endotracheal tube with stylet on successful intubation on the first attempt.
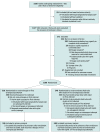
Design, setting, and participants: The Bougie or Stylet in Patients Undergoing Intubation Emergently (BOUGIE) trial was a multicenter, randomized clinical trial among 1102 critically ill adults undergoing tracheal intubation in 7 emergency departments and 8 intensive care units in the US between April 29, 2019, and February 14, 2021; the date of final follow-up was March 14, 2021.
Interventions: Patients were randomly assigned to use of a bougie (n = 556) or use of an endotracheal tube with stylet (n = 546).
Main outcomes and measures: The primary outcome was successful intubation on the first attempt. The secondary outcome was the incidence of severe hypoxemia, defined as a peripheral oxygen saturation less than 80%.
Results: Among 1106 patients randomized, 1102 (99.6%) completed the trial and were included in the primary analysis (median age, 58 years; 41.0% women). Successful intubation on the first attempt occurred in 447 patients (80.4%) in the bougie group and 453 patients (83.0%) in the stylet group (absolute risk difference, -2.6 percentage points [95% CI, -7.3 to 2.2]; P = .27). A total of 58 patients (11.0%) in the bougie group experienced severe hypoxemia, compared with 46 patients (8.8%) in the stylet group (absolute risk difference, 2.2 percentage points [95% CI, -1.6 to 6.0]). Esophageal intubation occurred in 4 patients (0.7%) in the bougie group and 5 patients (0.9%) in the stylet group, pneumothorax was present after intubation in 14 patients (2.5%) in the bougie group and 15 patients (2.7%) in the stylet group, and injury to oral, glottic, or thoracic structures occurred in 0 patients in the bougie group and 3 patients (0.5%) in the stylet group.
Conclusions and relevance: Among critically ill adults undergoing tracheal intubation, use of a bougie did not significantly increase the incidence of successful intubation on the first attempt compared with use of an endotracheal tube with stylet.
Trial registration: ClinicalTrials.gov Identifier: NCT03928925
Conflict of interest statement
Figures

Comment in
-
Use of a Bougie vs Endotracheal Tube With Stylet and Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation.JAMA. 2022 Apr 19;327(15):1503. doi: 10.1001/jama.2022.2713. JAMA. 2022. PMID: 35438735 No abstract available.
-
Use of a Bougie vs Endotracheal Tube With Stylet and Successful Intubation on the First Attempt Among Critically Ill Patients Undergoing Tracheal Intubation.JAMA. 2022 Apr 19;327(15):1502-1503. doi: 10.1001/jama.2022.2710. JAMA. 2022. PMID: 35438736 No abstract available.
References
-
- Pfuntner A, Wier LM, Stocks C. Most frequent procedures performed in US hospitals, 2011: statistical brief# 165. In: Agency for Healthcare Research and Quality , ed. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality; 2013:1-10.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

