Assembled Cell-Decorated Collagen (AC-DC) Fiber Bioprinted Implants with Musculoskeletal Tissue Properties Promote Functional Recovery in Volumetric Muscle Loss
- PMID: 34879177
- PMCID: PMC8890793
- DOI: 10.1002/adhm.202101357
Assembled Cell-Decorated Collagen (AC-DC) Fiber Bioprinted Implants with Musculoskeletal Tissue Properties Promote Functional Recovery in Volumetric Muscle Loss
Abstract
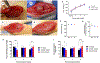
Musculoskeletal tissue injuries, including volumetric muscle loss (VML), are commonplace and often lead to permanent disability and deformation. Addressing this healthcare need, an advanced biomanufacturing platform, assembled cell-decorated collagen (AC-DC) bioprinting, is invented to rapidly and reproducibly create living biomaterial implants, using clinically relevant cells and strong, microfluidic wet-extruded collagen microfibers. Quantitative analysis shows that the directionality and distribution of cells throughout AC-DC implants mimic native musculoskeletal tissue. AC-DC bioprinted implants further approximate or exceed the strength and stiffness of human musculoskeletal tissue and exceed collagen hydrogel tensile properties by orders of magnitude. In vivo, AC-DC implants are assessed in a critically sized muscle injury in the hindlimb, with limb torque generation potential measured over 12 weeks. Both acellular and cellular implants promote functional recovery compared to the unrepaired group, with AC-DC implants containing therapeutic muscle progenitor cells promoting the highest degree of recovery. Histological analysis and automated image processing of explanted muscle cross-sections reveal increased total muscle fiber count, median muscle fiber size, and increased cellularization for injuries repaired with cellularized implants. These studies introduce an advanced bioprinting method for generating musculoskeletal tissue analogs with near-native biological and biomechanical properties with the potential to repair myriad challenging musculoskeletal injuries.
Keywords: AC-DC; bioprinting; collagen; microfibers; volumetric muscle loss.
© 2021 Wiley-VCH GmbH.
Figures



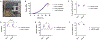


References
-
- Butler DL, Juncosa N, Dressler MR, Functional Efficacy of Tendon Repair Processes, Annual Review of Biomedical Engineering 6, 303–329 (2004). - PubMed
-
- Covey DC, Blast and Fragment Injuries of the Musculoskeletal System, JBJS 84, 1221–1234 (2002). - PubMed
-
- Janssen I, Heymsfield SB, Wang Z, Ross R, Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr, Journal of Applied Physiology 89, 81–88 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

