Protection against Covid-19 by BNT162b2 Booster across Age Groups
- PMID: 34879188
- PMCID: PMC8728796
- DOI: 10.1056/NEJMoa2115926
Protection against Covid-19 by BNT162b2 Booster across Age Groups
Abstract
Background: After promising initial results from the administration of a third (booster) dose of the BNT162b2 messenger RNA vaccine (Pfizer-BioNTech) to persons 60 years of age or older, the booster campaign in Israel was gradually expanded to persons in younger age groups who had received a second dose at least 5 months earlier.
Methods: We extracted data for the period from July 30 to October 10, 2021, from the Israel Ministry of Health database regarding 4,696,865 persons 16 years of age or older who had received two doses of BNT162b2 at least 5 months earlier. In the primary analysis, we compared the rates of confirmed coronavirus disease 2019 (Covid-19), severe illness, and death among those who had received a booster dose at least 12 days earlier (booster group) with the rates among those who had not received a booster (nonbooster group). In a secondary analysis, we compared the rates in the booster group with the rates among those who had received a booster 3 to 7 days earlier (early postbooster group). We used Poisson regression models to estimate rate ratios after adjusting for possible confounding factors.
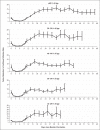
Results: The rate of confirmed infection was lower in the booster group than in the nonbooster group by a factor of approximately 10 (range across five age groups, 9.0 to 17.2) and was lower in the booster group than in the early postbooster group by a factor of 4.9 to 10.8. The adjusted rate difference ranged from 57.0 to 89.5 infections per 100,000 person-days in the primary analysis and from 34.4 to 38.3 in the secondary analysis. The rates of severe illness in the primary and secondary analyses were lower in the booster group by a factor of 17.9 (95% confidence interval [CI], 15.1 to 21.2) and 6.5 (95% CI, 5.1 to 8.2), respectively, among those 60 years of age or older and by a factor of 21.7 (95% CI, 10.6 to 44.2) and 3.7 (95% CI, 1.3 to 10.2) among those 40 to 59 years of age. The adjusted rate difference in the primary and secondary analyses was 5.4 and 1.9 cases of severe illness per 100,000 person-days among those 60 years of age or older and 0.6 and 0.1 among those 40 to 59 years of age. Among those 60 years of age or older, mortality was lower by a factor of 14.7 (95% CI, 10.0 to 21.4) in the primary analysis and 4.9 (95% CI, 3.1 to 7.9) in the secondary analysis. The adjusted rate difference in the primary and secondary analyses was 2.1 and 0.8 deaths per 100,000 person-days.
Conclusions: Across the age groups studied, rates of confirmed Covid-19 and severe illness were substantially lower among participants who received a booster dose of the BNT162b2 vaccine than among those who did not.
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Booster Doses and Prioritizing Lives Saved.N Engl J Med. 2021 Dec 23;385(26):2476-2477. doi: 10.1056/NEJMe2117592. Epub 2021 Dec 8. N Engl J Med. 2021. PMID: 34879192 Free PMC article. No abstract available.
References
-
- Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. August 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1). preprint.
-
- Patalon T, Gazit S, Pitzer VE, et al. Short term reduction in the odds of testing positive for SARS-CoV-2; a comparison between two doses and three doses of the BNT162b2 vaccine. August 31, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.29.21262792v1). preprint.
-
- COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. Bethesda, MD: National Institutes of Health, 2021 (https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
