BNT162b2 Vaccine Booster and Mortality Due to Covid-19
- PMID: 34879190
- PMCID: PMC8728797
- DOI: 10.1056/NEJMoa2115624
BNT162b2 Vaccine Booster and Mortality Due to Covid-19
Abstract
Background: The emergence of the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 and the reduced effectiveness over time of the BNT162b2 vaccine (Pfizer-BioNTech) led to a resurgence of coronavirus disease 2019 (Covid-19) cases in populations that had been vaccinated early. On July 30, 2021, the Israeli Ministry of Health approved the use of a third dose of BNT162b2 (booster) to cope with this resurgence. Evidence regarding the effectiveness of the booster in lowering mortality due to Covid-19 is still needed.
Methods: We obtained data for all members of Clalit Health Services who were 50 years of age or older at the start of the study and had received two doses of BNT162b2 at least 5 months earlier. The mortality due to Covid-19 among participants who received the booster during the study period (booster group) was compared with that among participants who did not receive the booster (nonbooster group). A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of booster status with death due to Covid-19, with adjustment for sociodemographic factors and coexisting conditions.
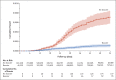
Results: A total of 843,208 participants met the eligibility criteria, of whom 758,118 (90%) received the booster during the 54-day study period. Death due to Covid-19 occurred in 65 participants in the booster group (0.16 per 100,000 persons per day) and in 137 participants in the nonbooster group (2.98 per 100,000 persons per day). The adjusted hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% confidence interval, 0.07 to 0.14; P<0.001).
Conclusions: Participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 than participants who did not receive a booster.
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Booster Doses and Prioritizing Lives Saved.N Engl J Med. 2021 Dec 23;385(26):2476-2477. doi: 10.1056/NEJMe2117592. Epub 2021 Dec 8. N Engl J Med. 2021. PMID: 34879192 Free PMC article. No abstract available.
-
BNT162b2 Vaccine Booster and Covid-19 Mortality.N Engl J Med. 2022 Mar 10;386(10):1000. doi: 10.1056/NEJMc2120044. Epub 2022 Feb 9. N Engl J Med. 2022. PMID: 35139266 No abstract available.
-
BNT162b2 Vaccine Booster and Covid-19 Mortality.N Engl J Med. 2022 Mar 10;386(10):1000. doi: 10.1056/NEJMc2120044. Epub 2022 Feb 9. N Engl J Med. 2022. PMID: 35139267 No abstract available.
-
Potential "Healthy Vaccinee Bias" in a Study of BNT162b2 Vaccine against Covid-19.N Engl J Med. 2023 Jul 20;389(3):284-285. doi: 10.1056/NEJMc2306683. N Engl J Med. 2023. PMID: 37470285 No abstract available.
-
Potential "Healthy Vaccinee Bias" in a Study of BNT162b2 Vaccine against Covid-19. Reply.N Engl J Med. 2023 Jul 20;389(3):285-286. doi: 10.1056/NEJMc2306683. N Engl J Med. 2023. PMID: 37470347 No abstract available.
References
-
- Daily new confirmed COVID-19 cases per million people. Our World In Data. September 22, 2021. (https://ourworldindata.org/covid-cases?country=IND~USA~GBR~CAN~DEU~FRA~I...).
-
- Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. August 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1). preprint. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous