The effect of limb position on a static knee extension task can be explained with a simple spinal cord circuit model
- PMID: 34879209
- PMCID: PMC8802899
- DOI: 10.1152/jn.00208.2021
The effect of limb position on a static knee extension task can be explained with a simple spinal cord circuit model
Abstract
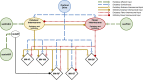
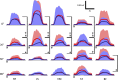
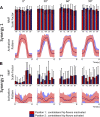
The influence of proprioceptive feedback on muscle activity during isometric tasks is the subject of conflicting studies. We performed an isometric knee extension task experiment based on two common clinical tests for mobility and flexibility. The task was carried out at four preset angles of the knee, and we recorded from five muscles for two different hip positions. We applied muscle synergy analysis using nonnegative matrix factorization on surface electromyograph recordings to identify patterns in the data that changed with internal knee angle, suggesting a link between proprioception and muscle activity. We hypothesized that such patterns arise from the way proprioceptive and cortical signals are integrated in neural circuits of the spinal cord. Using the MIIND neural simulation platform, we developed a computational model based on current understanding of spinal circuits with an adjustable afferent input. The model produces the same synergy trends as observed in the data, driven by changes in the afferent input. To match the activation patterns from each knee angle and position of the experiment, the model predicts the need for three distinct inputs: two to control a nonlinear bias toward the extensors and against the flexors, and a further input to control additional inhibition of rectus femoris. The results show that proprioception may be involved in modulating muscle synergies encoded in cortical or spinal neural circuits.NEW & NOTEWORTHY The role of sensory feedback in motor control when limbs are held in a fixed position is disputed. We performed a novel experiment involving fixed position tasks based on two common clinical tests. We identified patterns of muscle activity during the tasks that changed with different leg positions and then inferred how sensory feedback might influence the observations. We developed a computational model that required three distinct inputs to reproduce the activity patterns observed experimentally. The model provides a neural explanation for how the activity patterns can be changed by sensory feedback.
Keywords: isometric knee extension; neural control; population model; proprioception; spinal circuits.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

