Ischemic-Trained Monocytes Improve Arteriogenesis in a Mouse Model of Hindlimb Ischemia
- PMID: 34879707
- PMCID: PMC8792358
- DOI: 10.1161/ATVBAHA.121.317197
Ischemic-Trained Monocytes Improve Arteriogenesis in a Mouse Model of Hindlimb Ischemia
Abstract
Objective: Monocytes, which play an important role in arteriogenesis, can build immunologic memory by a functional reprogramming that modifies their response to a second challenge. This process, called trained immunity, is evoked by insults that shift monocyte metabolism, increasing HIF (hypoxia-inducible factor)-1α levels. Since ischemia enhances HIF-1α, we evaluate whether ischemia can lead to a functional reprogramming of monocytes, which would contribute to arteriogenesis after hindlimb ischemia.
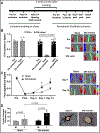
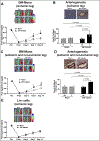
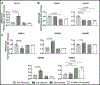
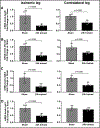
Methods and results: Mice exposed to ischemia by 24 hours (24h) of femoral artery occlusion (24h trained) or sham were subjected to hindlimb ischemia one week later; the 24h trained mice showed significant improvement in blood flow recovery and arteriogenesis after hindlimb ischemia. Adoptive transfer using bone marrow-derived monocytes (BM-Mono) from 24h trained or sham donor mice, demonstrated that recipients subjected to hindlimb ischemia who received 24h ischemic-trained monocytes had remarkable blood flow recovery and arteriogenesis. Further, ischemic-trained BM-Mono had increased HIF-1α and GLUT-1 (glucose transporter-1) gene expression during femoral artery occlusion. Circulating cytokines and GLUT-1 were also upregulated during femoral artery occlusion.Transcriptomic analysis and confirmatory qPCR performed in 24h trained and sham BM-Mono revealed that among the 15 top differentially expressed genes, 4 were involved in lipid metabolism in the ischemic-trained monocytes. Lipidomic analysis confirmed that ischemia training altered the cholesterol metabolism of these monocytes. Further, several histone-modifying epigenetic enzymes measured by qPCR were altered in mouse BM-Mono exposed to 24h hypoxia.
Conclusions: Ischemia training in BM-Mono leads to a unique gene profile and improves blood flow and arteriogenesis after hindlimb ischemia.
Keywords: bone marrow; hindlimb; ischemia; lipids; monocytes.
Figures

Comment in
-
Break on Through to the Other Side: How Trained Monocytes Promote Recovery From Hind Limb Ischemia.Arterioscler Thromb Vasc Biol. 2022 Feb;42(2):189-192. doi: 10.1161/ATVBAHA.121.317257. Epub 2021 Dec 23. Arterioscler Thromb Vasc Biol. 2022. PMID: 34937387 Free PMC article. No abstract available.
References
-
- Scholz D, Ziegelhoeffer T, Helisch A, et al. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. - PubMed
-
- Kinnaird T, Stabile E, Burnett MS, et al. Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote In Vitro and In Vivo Arteriogenesis Through Paracrine Mechanisms. Circ Res. 2004;94:678–685. doi:10.1161/01.RES.0000118601.37875.AC - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

