Efficacy and Complications of Subcutaneous versus Conventional Cardioverter Defibrillators: A Systematic Review and Meta-analysis
- PMID: 34879809
- PMCID: PMC9615219
- DOI: 10.2174/1573403X17666211208100151
Efficacy and Complications of Subcutaneous versus Conventional Cardioverter Defibrillators: A Systematic Review and Meta-analysis
Abstract
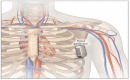
Background/objectives: Implantable cardioverter defibrillators are used to prevent sudden cardiac death. The subcutaneous implantable cardioverter-defibrillator was newly developed to overcome the limitations of the conventional implantable cardioverter defibrillator-transvenous device. The subcutaneous implantable cardioverter defibrillator is indicated for young patients with heart disease, congenital heart defects, and poor venous access, who have an indication for implantable cardioverter defibrillator without the need for anti-bradycardic stimulation. We aimed to compare the efficacy and complications of subcutaneous with transvenous implantable cardioverter- defibrillator devices.
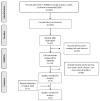
Methodology: A systematic review was conducted using different databases. The inclusion criteria were observational and clinical randomized trials with no language limits and no publication date limit that compared subcutaneous with transvenous implantable cardioverter-defibrillators. The selected patients were aged > 18 years with complex ventricular arrhythmia.
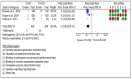
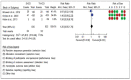
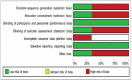
Results: Five studies involving 2111 patients who underwent implantable cardioverter defibrillator implantation were included. The most frequent complication in the subcutaneous device group was infection, followed by hematoma formation and electrode migration. For the transvenous device, the most frequent complications were electrode migration and infection. Regarding efficacy, the total rates of appropriate shocks were 9.04% and 20.47% in the subcutaneous and transvenous device groups, respectively, whereas inappropriate shocks to the subcutaneous and transvenous device groups were 11,3% and 10,7%, respectively.
Conclusion: When compared to the transvenous device, the subcutaneous device had lower complication rates owing to lead migration and less inappropriate shocks due to supraventricular tachycardia; nevertheless, infection rates and improper shocks due to T wave oversensing were comparable for both devices CRD42021251569.
Keywords: Subcutaneous implantable cardioverter defibrillator; bruise; complications; efficacy; infection; supraventricular tachycardia; transvenous implantable cardioverter defibrillator.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
