Altitude acclimatization, hemoglobin-oxygen affinity, and circulatory oxygen transport in hypoxia
- PMID: 34879970
- PMCID: PMC8821351
- DOI: 10.1016/j.mam.2021.101052
Altitude acclimatization, hemoglobin-oxygen affinity, and circulatory oxygen transport in hypoxia
Abstract
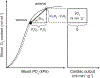
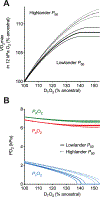
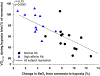
In mammals and other air-breathing vertebrates that live at high altitude, adjustments in convective O2 transport via changes in blood hemoglobin (Hb) content and/or Hb-O2 affinity can potentially mitigate the effects of arterial hypoxemia. However, there are conflicting views about the optimal values of such traits in hypoxia, partly due to the intriguing observation that hypoxia-induced acclimatization responses in humans and other predominantly lowland mammals are frequently not aligned in the same direction as evolved phenotypic changes in high-altitude natives. Here we review relevant theoretical and empirical results and we highlight experimental studies of rodents and humans that provide insights into the combination of hematological changes that help attenuate the decline in aerobic performance in hypoxia. For a given severity of hypoxia, experimental results suggest that optimal values for hematological traits are conditional on the states of other interrelated phenotypes that govern different steps in the O2-transport pathway.
Keywords: Aerobic performance; High-altitude adaptation; Hypoxia; Oxygen transport pathway; VO(2max).
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest: none
Figures
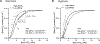


References
-
- Aste-Salazar H, Hurtado A. 1944. The affinity of hemoglobin for oxygen at sea level and at high altitudes. American Journal of Physiology 142, 733–743.
-
- Bencowitz HZ, Wagner PD, West JB. 1982. Effect of change in P50 on exercise tolerance at high-altitude - a theoretical study. Journal of Applied Physiology 53, 1487–1495. - PubMed
-
- Bouverot P 1985. Adaptation to Altitude-Hypoxia in Vertebrates. Berlin: Springer-Verlag.
-
- Calbet JAL, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. 2003. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? American Journal of Physiology-Regulatory Integrative and Comparative Physiology 284, R304–R316. - PubMed
-
- Calbet JAL, Lundby C, Koskolou M, Boushel R. 2006. Importance of hemoglobin concentration to exercise: Acute manipulations. Respiratory Physiology & Neurobiology 151, 132–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

