Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome
- PMID: 34880074
- PMCID: PMC8819987
- DOI: 10.1681/ASN.2021050643
Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome
Erratum in
-
Correction: Mycophenolate Mofetil after Rituximab for Childhood-Onset Complicated Frequently-Relapsing or Steroid-Dependent Nephrotic Syndrome.J Am Soc Nephrol. 2022 May;33(5):1050. doi: 10.1681/ASN.2022020134. J Am Soc Nephrol. 2022. PMID: 35487689 Free PMC article. No abstract available.
Abstract
Background: Rituximab is the standard therapy for childhood-onset complicated frequently relapsing or steroid-dependent nephrotic syndrome (FRNS/SDNS). However, most patients redevelop FRNS/SDNS after peripheral B cell recovery.
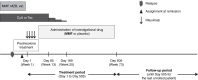
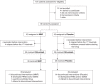
Methods: We conducted a multicenter, randomized, double-blind, placebo-controlled trial to examine whether mycophenolate mofetil (MMF) administration after rituximab can prevent treatment failure (FRNS, SDNS, steroid resistance, or use of immunosuppressive agents or rituximab). In total, 39 patients (per group) were treated with rituximab, followed by either MMF or placebo until day 505 (treatment period). The primary outcome was time to treatment failure (TTF) throughout the treatment and follow-up periods (until day 505 for the last enrolled patient).
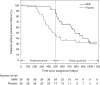
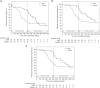
Results: TTFs were clinically but not statistically significantly longer among patients given MMF after rituximab than among patients receiving rituximab monotherapy (median, 784.0 versus 472.5 days, hazard ratio [HR], 0.59; 95% confidence interval [95% CI], 0.34 to 1.05, log-rank test: P=0.07). Because most patients in the MMF group presented with treatment failure after MMF discontinuation, we performed a post-hoc analysis limited to the treatment period and found that MMF after rituximab prolonged the TTF and decreased the risk of treatment failure by 80% (HR, 0.20; 95% CI, 0.08 to 0.50). Moreover, MMF after rituximab reduced the relapse rate and daily steroid dose during the treatment period by 74% and 57%, respectively. The frequency and severity of adverse events were similar in both groups.
Conclusions: Administration of MMF after rituximab may sufficiently prevent the development of treatment failure and is well tolerated, although the relapse-preventing effect disappears after MMF discontinuation.
Keywords: childhood-onset; clinical trial; complicated frequently-relapsing/steroid-dependent nephrotic syndrome; mycophenolate mofetil; rituximab.
Copyright © 2022 by the American Society of Nephrology.
Figures

References
-
- International Study of Kidney Disease in Children : Nephrotic syndrome in children: Prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13: 159–165, 1978 - PubMed
-
- Kikunaga K, Ishikura K, Terano C, Sato M, Komaki F, Hamasaki Y, et al. ; Japanese Pediatric Survey Holding Information of NEphrotic syndrome (JP-SHINE) study of the Japanese Study Group of Renal Disease in Children : High incidence of idiopathic nephrotic syndrome in East Asian children: a nationwide survey in Japan (JP-SHINE study). Clin Exp Nephrol 21: 651–657, 2017 - PubMed
-
- International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 - PubMed
-
- Noone DG, Iijima K, Parekh R: Idiopathic nephrotic syndrome in children. Lancet 392: 61–74, 2018 - PubMed
-
- Kidney Disease Improving Global Outcomes (KDIGO): Clinical Practice Guideline for the Management of Glomerular Diseases: Chapter 4: Nephrotic syndrome in children. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Glomerular-Diseases-G.... Accessed May 1, 2021
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

