The hippocampus as the switchboard between perception and memory
- PMID: 34880133
- PMCID: PMC8685930
- DOI: 10.1073/pnas.2114171118
The hippocampus as the switchboard between perception and memory
Abstract
Adaptive memory recall requires a rapid and flexible switch from external perceptual reminders to internal mnemonic representations. However, owing to the limited temporal or spatial resolution of brain imaging modalities used in isolation, the hippocampal-cortical dynamics supporting this process remain unknown. We thus employed an object-scene cued recall paradigm across two studies, including intracranial electroencephalography (iEEG) and high-density scalp EEG. First, a sustained increase in hippocampal high gamma power (55 to 110 Hz) emerged 500 ms after cue onset and distinguished successful vs. unsuccessful recall. This increase in gamma power for successful recall was followed by a decrease in hippocampal alpha power (8 to 12 Hz). Intriguingly, the hippocampal gamma power increase marked the moment at which extrahippocampal activation patterns shifted from perceptual cue toward mnemonic target representations. In parallel, source-localized EEG alpha power revealed that the recall signal progresses from hippocampus to posterior parietal cortex and then to medial prefrontal cortex. Together, these results identify the hippocampus as the switchboard between perception and memory and elucidate the ensuing hippocampal-cortical dynamics supporting the recall process.
Keywords: gamma power; hippocampus; intracranial EEG; memory; recall.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

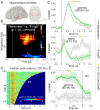
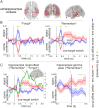
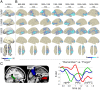
References
-
- Lisman J. E., Relating hippocampal circuitry to function: Recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron 22, 233–242 (1999). - PubMed
-
- Wallenstein G. V., Eichenbaum H., Hasselmo M. E., The hippocampus as an associator of discontiguous events. Trends Neurosci. 21, 317–323 (1998). - PubMed
-
- Teyler T. J., DiScenna P., The hippocampal memory indexing theory. Behav. Neurosci. 100, 147–154 (1986). - PubMed
-
- Marr D., Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

