Helicase Q promotes homology-driven DNA double-strand break repair and prevents tandem duplications
- PMID: 34880204
- PMCID: PMC8654963
- DOI: 10.1038/s41467-021-27408-z
Helicase Q promotes homology-driven DNA double-strand break repair and prevents tandem duplications
Abstract
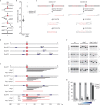
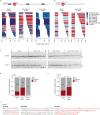
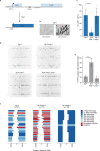
DNA double-strand breaks are a major threat to cellular survival and genetic integrity. In addition to high fidelity repair, three intrinsically mutagenic DNA break repair routes have been described, i.e. single-strand annealing (SSA), polymerase theta-mediated end-joining (TMEJ) and residual ill-defined microhomology-mediated end-joining (MMEJ) activity. Here, we identify C. elegans Helicase Q (HELQ-1) as being essential for MMEJ as well as for SSA. We also find HELQ-1 to be crucial for the synthesis-dependent strand annealing (SDSA) mode of homologous recombination (HR). Loss of HELQ-1 leads to increased genome instability: patchwork insertions arise at deletion junctions due to abortive rounds of polymerase theta activity, and tandem duplications spontaneously accumulate in genomes of helq-1 mutant animals as a result of TMEJ of abrogated HR intermediates. Our work thus implicates HELQ activity for all DSB repair modes guided by complementary base pairs and provides mechanistic insight into mutational signatures common in HR-defective cancers.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Helicase HELQ: Molecular Characters Fit for DSB Repair Function.Int J Mol Sci. 2024 Aug 8;25(16):8634. doi: 10.3390/ijms25168634. Int J Mol Sci. 2024. PMID: 39201320 Free PMC article. Review.
-
Meiotic Double-Strand Break Proteins Influence Repair Pathway Utilization.Genetics. 2018 Nov;210(3):843-856. doi: 10.1534/genetics.118.301402. Epub 2018 Sep 21. Genetics. 2018. PMID: 30242011 Free PMC article.
-
Single-strand annealing mediates the conservative repair of double-strand DNA breaks in homologous recombination-defective germ cells of Caenorhabditis elegans.DNA Repair (Amst). 2019 Mar;75:18-28. doi: 10.1016/j.dnarep.2019.01.007. Epub 2019 Jan 24. DNA Repair (Amst). 2019. PMID: 30710866
-
Division of Labor by the HELQ, BLM, and FANCM Helicases during Homologous Recombination Repair in Drosophila melanogaster.Genes (Basel). 2022 Mar 8;13(3):474. doi: 10.3390/genes13030474. Genes (Basel). 2022. PMID: 35328029 Free PMC article.
-
Contribution of Microhomology to Genome Instability: Connection between DNA Repair and Replication Stress.Int J Mol Sci. 2022 Oct 26;23(21):12937. doi: 10.3390/ijms232112937. Int J Mol Sci. 2022. PMID: 36361724 Free PMC article. Review.
Cited by
-
FAN1-mediated translesion synthesis and POLQ/HELQ-mediated end joining generate interstrand crosslink-induced mutations.Nat Commun. 2025 Mar 13;16(1):2495. doi: 10.1038/s41467-025-57764-z. Nat Commun. 2025. PMID: 40082407 Free PMC article.
-
Helicase HELQ: Molecular Characters Fit for DSB Repair Function.Int J Mol Sci. 2024 Aug 8;25(16):8634. doi: 10.3390/ijms25168634. Int J Mol Sci. 2024. PMID: 39201320 Free PMC article. Review.
-
POLQ suppresses genome instability and alterations in DNA repeat tract lengths.NAR Cancer. 2022 Jun 29;4(3):zcac020. doi: 10.1093/narcan/zcac020. eCollection 2022 Sep. NAR Cancer. 2022. PMID: 35774233 Free PMC article.
-
Experimental systems for the analysis of mutational signatures: no 'one-size-fits-all' solution.Biochem Soc Trans. 2023 Jun 28;51(3):1307-1317. doi: 10.1042/BST20221482. Biochem Soc Trans. 2023. PMID: 37283472 Free PMC article.
-
POLD3 as Controller of Replicative DNA Repair.Int J Mol Sci. 2024 Nov 19;25(22):12417. doi: 10.3390/ijms252212417. Int J Mol Sci. 2024. PMID: 39596481 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

