Essential role of a Plasmodium berghei heat shock protein (PBANKA_0938300) in gametocyte development
- PMID: 34880324
- PMCID: PMC8654831
- DOI: 10.1038/s41598-021-03059-4
Essential role of a Plasmodium berghei heat shock protein (PBANKA_0938300) in gametocyte development
Abstract
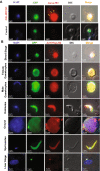
The continued existence of Plasmodium parasites in physiologically distinct environments during their transmission in mosquitoes and vertebrate hosts requires effector proteins encoded by parasite genes to provide adaptability. Parasites utilize their robust stress response system involving heat shock proteins for their survival. Molecular chaperones are involved in maintaining protein homeostasis within a cell during stress, protein biogenesis and the formation of protein complexes. Due to their critical role in parasite virulence, they are considered targets for therapeutic interventions. Our results identified a putative P. berghei heat shock protein (HSP) belonging to the HSP40 family (HspJ62), which is abundantly induced upon heat stress and expressed during all parasite stages. To determine the role HspJ62, a gene-disrupted P. berghei transgenic line was developed (ΔHspJ62), which resulted in disruption of gametocyte formation. Such parasites were unable to form subsequent sexual stages because of disrupted gametogenesis, indicating the essential role of HspJ62 in gametocyte formation. Transcriptomic analysis of the transgenic line showed downregulation of a number of genes, most of which were specific to male or female gametocytes. The transcription factor ApiAP2 was also downregulated in ΔHspJ62 parasites. Our findings suggest that the downregulation of ApiAP2 likely disrupts the transcriptional regulation of sexual stage genes, leading to impaired gametogenesis. This finding also highlights the critical role that HspJ62 indirectly plays in the development of P. berghei sexual stages and in facilitating the conversion from the asexual blood stage to the sexual stage. This study characterizes the HspJ62 protein as a fertility factor because parasites lacking it are unable to transmit to mosquitoes. This study adds an important contribution to ongoing research aimed at understanding gametocyte differentiation and formation in parasites. The molecule adds to the list of potential drug targets that can be targeted to inhibit parasite sexual development and consequently parasite transmission.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Comparative proteomic analysis of kinesin-8B deficient Plasmodium berghei during gametogenesis.J Proteomics. 2021 Mar 30;236:104118. doi: 10.1016/j.jprot.2021.104118. Epub 2021 Jan 21. J Proteomics. 2021. PMID: 33486016
-
A G-Protein-Coupled Receptor Modulates Gametogenesis via PKG-Mediated Signaling Cascade in Plasmodium berghei.Microbiol Spectr. 2022 Apr 27;10(2):e0015022. doi: 10.1128/spectrum.00150-22. Epub 2022 Apr 11. Microbiol Spectr. 2022. PMID: 35404079 Free PMC article.
-
Dynamic molecular events associated to Plasmodium berghei gametogenesis through proteomic approach.J Proteomics. 2018 May 30;180:88-98. doi: 10.1016/j.jprot.2017.11.009. Epub 2017 Nov 15. J Proteomics. 2018. PMID: 29155091
-
Sexual development in Plasmodium parasites: knowing when it's time to commit.Nat Rev Microbiol. 2015 Sep;13(9):573-87. doi: 10.1038/nrmicro3519. Nat Rev Microbiol. 2015. PMID: 26272409 Review.
-
Plasmodium falciparum gametocytes: still many secrets of a hidden life.Mol Microbiol. 2007 Oct;66(2):291-302. doi: 10.1111/j.1365-2958.2007.05904.x. Epub 2007 Sep 3. Mol Microbiol. 2007. PMID: 17784927 Review.
Cited by
-
Role of inflammasomes in cancer immunity: mechanisms and therapeutic potential.J Exp Clin Cancer Res. 2025 Mar 29;44(1):109. doi: 10.1186/s13046-025-03366-y. J Exp Clin Cancer Res. 2025. PMID: 40155968 Free PMC article. Review.
-
Toxoplasma gondii bradyzoite-specific BAG1 is nonessential for cyst formation due to compensation by other heat-shock proteins.Parasit Vectors. 2024 Jul 30;17(1):322. doi: 10.1186/s13071-024-06339-w. Parasit Vectors. 2024. PMID: 39080770 Free PMC article.
-
Therapeutic mucosal vaccination of herpes simplex virus type 2 infected guinea pigs with an adenovirus-based vaccine expressing the ribonucleotide reductase 2 and glycoprotein D induces local tissue-resident CD4+ and CD8+ TRM cells associated with protection against recurrent genital herpes.Front Immunol. 2025 Mar 26;16:1568258. doi: 10.3389/fimmu.2025.1568258. eCollection 2025. Front Immunol. 2025. PMID: 40207227 Free PMC article.
-
Therapeutic prime/pull vaccination of HSV-2-infected guinea pigs with the ribonucleotide reductase 2 (RR2) protein and CXCL11 chemokine boosts antiviral local tissue-resident and effector memory CD4+ and CD8+ T cells and protects against recurrent genital herpes.J Virol. 2024 May 14;98(5):e0159623. doi: 10.1128/jvi.01596-23. Epub 2024 Apr 8. J Virol. 2024. PMID: 38587378 Free PMC article.
-
A Spike-Based mRNA Vaccine Encapsulated in Phospholipid 1,2-Dioleoyl-sn-Glycero-3-PhosphoEthanolamine Containing Lipid Nanoparticles Induced Potent B- and T-Cell Responses Associated with Protection Against SARS-CoV-2 Infection and COVID-19-like Symptoms in Hamsters.Vaccines (Basel). 2025 Jan 8;13(1):47. doi: 10.3390/vaccines13010047. Vaccines (Basel). 2025. PMID: 39852826 Free PMC article.
References
-
- World Health Organization . World Malaria Report 2019. WHO; 2019.
-
- Rudzinska MA. The fine structure of malaria parasites. Int. Rev. Cytol. 1969;25:161–199. - PubMed
-
- Nkuo-Akenji T, et al. Environmental factors affecting malaria parasite prevalence in rural Bolifamba, South West Cameroon. Afr. J. Health Sci. 2006;13:40–46. - PubMed
-
- Beere HM, Green DR. Stress management: Heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001;11:6–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

