PET imaging of brain aromatase in humans and rhesus monkeys by 11C-labeled cetrozole analogs
- PMID: 34880350
- PMCID: PMC8654920
- DOI: 10.1038/s41598-021-03063-8
PET imaging of brain aromatase in humans and rhesus monkeys by 11C-labeled cetrozole analogs
Abstract
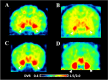
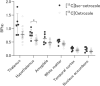
Aromatase is an estrogen synthetic enzyme that plays important roles in brain functions. To quantify aromatase expression in the brain by positron emission tomography (PET), we had previously developed [11C]cetrozole, which showed high specificity and affinity. To develop more efficient PET tracer(s) for aromatase imaging, we synthesized three analogs of cetrozole. We synthesized meta-cetrozole, nitro-cetrozole, and iso-cetrozole, and prepared the corresponding 11C-labeled tracers. The inhibitory activities of these three analogs toward aromatase were evaluated using marmoset placenta, and PET imaging of brain aromatase was performed using the 11C-labeled tracers in monkeys. The most promising analog in the monkey study, iso-cetrozole, was evaluated in the human PET study. The highest to lowest inhibitory activity of the analogs toward aromatase in the microsomal fraction from marmoset placenta was in the following order: iso-cetrozole, nitro-cetrozole, cetrozole, and meta-cetrozole. This order showed good agreement with the order of the binding potential (BP) of each 11C-labeled analog to aromatase in the rhesus monkey brain. A human PET study using [11C]iso-analog showed a similar distribution pattern of binding as that of [11C]cetrozole. The time-activity curves showed that elimination of [11C]iso-cetrozole from brain tissue was faster than that of 11C-cetrozole, indicating more rapid metabolism of [11C]iso-cetrozole. [11C]Cetrozole has preferable metabolic stability for brain aromatase imaging in humans, although [11C]iso-cetrozole might also be useful to measure aromatase level in living human brain because of its high binding potential.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
11C-cetrozole: an improved C-11C-methylated PET probe for aromatase imaging in the brain.J Nucl Med. 2014 May;55(5):852-7. doi: 10.2967/jnumed.113.131474. Epub 2014 Mar 27. J Nucl Med. 2014. PMID: 24676756
-
Quantification of aromatase binding in the female human brain using [11 C]cetrozole positron emission tomography.J Neurosci Res. 2020 Nov;98(11):2208-2218. doi: 10.1002/jnr.24707. Epub 2020 Aug 6. J Neurosci Res. 2020. PMID: 32761874
-
Comparison of pharmacokinetics of newly discovered aromatase inhibitors by a cassette microdosing approach in healthy Japanese subjects.Drug Metab Pharmacokinet. 2017 Dec;32(6):293-300. doi: 10.1016/j.dmpk.2017.09.003. Epub 2017 Sep 21. Drug Metab Pharmacokinet. 2017. PMID: 29137842 Clinical Trial.
-
Imaging of aromatase distribution in rat and rhesus monkey brains with [11C]vorozole.Nucl Med Biol. 2006 Jul;33(5):599-605. doi: 10.1016/j.nucmedbio.2006.03.009. Epub 2006 Jun 12. Nucl Med Biol. 2006. PMID: 16843834
-
In vivo visualization of aromatase in animals and humans.Front Neuroendocrinol. 2016 Jan;40:42-51. doi: 10.1016/j.yfrne.2015.10.001. Epub 2015 Oct 9. Front Neuroendocrinol. 2016. PMID: 26456904 Free PMC article. Review.
Cited by
-
Utilisation of the Innovative [18F]-Labelled Radiotracer [18F]-BIBD-071 Within HR+ Breast Cancer Xenograft Mouse Models.Pharmaceuticals (Basel). 2025 Jan 9;18(1):66. doi: 10.3390/ph18010066. Pharmaceuticals (Basel). 2025. PMID: 39861129 Free PMC article.
-
The historical progression of positron emission tomography research in neuroendocrinology.Front Neuroendocrinol. 2023 Jul;70:101081. doi: 10.1016/j.yfrne.2023.101081. Epub 2023 Jul 7. Front Neuroendocrinol. 2023. PMID: 37423505 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

