Ectomycorrhizal fungus supports endogenous rhythmic growth and corresponding resource allocation in oak during various below- and aboveground biotic interactions
- PMID: 34880358
- PMCID: PMC8654951
- DOI: 10.1038/s41598-021-03132-y
Ectomycorrhizal fungus supports endogenous rhythmic growth and corresponding resource allocation in oak during various below- and aboveground biotic interactions
Abstract
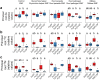
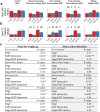
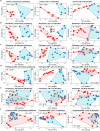
Endogenous rhythmic growth (ERG) is displayed by many tropical and some major temperate tree species and characterized by alternating root and shoot flushes (RF and SF). These flushes occur parallel to changes in biomass partitioning and in allocation of recently assimilated carbon and nitrogen. To address how biotic interactions interplay with ERG, we cross-compared the RF/SF shifts in oak microcuttings in the presence of pathogens, consumers and a mycorrhiza helper bacterium, without and with an ectomycorrhizal fungus (EMF), and present a synthesis of the observations. The typical increase in carbon allocation to sink leaves during SF did not occur in the presence of root or leaf pathogens, and the increase in nitrogen allocation to lateral roots during RF did not occur with the pathogens. The RF/SF shifts in resource allocation were mostly restored upon additional interaction with the EMF. Its presence led to increased resource allocation to principal roots during RF, also when the oaks were inoculated additionally with other interactors. The interactors affected the alternating, rhythmic growth and resource allocation shifts between shoots and roots. The restoring role of the EMF on RF/SF changes in parallel to the corresponding enhanced carbon and nitrogen allocation to sink tissues suggests that the EMF is supporting plants in maintaining the ERG.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Coley PD, Bryant JP, Chapin FS. Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. - PubMed
-
- Schaarschmidt S, Roitsch T, Hause B. Arbuscular mycorrhiza induces gene expression of the apoplastic invertase LIN6 in tomato (Lycopersicon esculentum) roots. J. Exp. Bot. 2006;57:4015–4023. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

