Structure and mechanism of the SGLT family of glucose transporters
- PMID: 34880492
- PMCID: PMC9482448
- DOI: 10.1038/s41586-021-04211-w
Structure and mechanism of the SGLT family of glucose transporters
Abstract
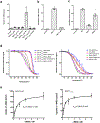
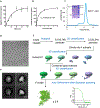
Glucose is a primary energy source in living cells. The discovery in 1960s that a sodium gradient powers the active uptake of glucose in the intestine1 heralded the concept of a secondary active transporter that can catalyse the movement of a substrate against an electrochemical gradient by harnessing energy from another coupled substrate. Subsequently, coupled Na+/glucose transport was found to be mediated by sodium-glucose cotransporters2,3 (SGLTs). SGLTs are responsible for active glucose and galactose absorption in the intestine and for glucose reabsorption in the kidney4, and are targeted by multiple drugs to treat diabetes5. Several members within the SGLT family transport key metabolites other than glucose2. Here we report cryo-electron microscopy structures of the prototypic human SGLT1 and a related monocarboxylate transporter SMCT1 from the same family. The structures, together with molecular dynamics simulations and functional studies, define the architecture of SGLTs, uncover the mechanism of substrate binding and selectivity, and shed light on water permeability of SGLT1. These results provide insights into the multifaceted functions of SGLTs.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures








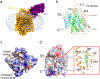


Comment in
-
Transporter-protein structures show how salt gets a sweet ride into cells.Nature. 2022 Jan;601(7892):194-196. doi: 10.1038/d41586-021-03555-7. Nature. 2022. PMID: 34880482 No abstract available.
References
-
- Kleinzeller A & Kotyk A Membrane transport and metabolism. (Praha: Publishing House of the Czechoslovak Academy of Sciences, 1961).
-
- Wright EM, Loo DDF & Hirayama BA Biology of human sodium glucose transporters. Physiol. Rev 91, 733–794 (2011). - PubMed
-
- Hopfer U, Nelson K & Isselbacher KJ Specific glucose transport in isolated brush border membranes from rat small-intestine. J. Biol. Chem 248, 25–32 (1973). - PubMed
-
- Wright EM Renal Na+-glucose cotransporters. Am. J. Physiol. Renal Physiol 280, F10–F18 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

